Organic Chemistry

Unit 3: Organic Chemistry Reactions
Combustion
All organic families will undergo complete and incomplete combustion
When there is an amine or amide, you will get nitrogen oxide as well
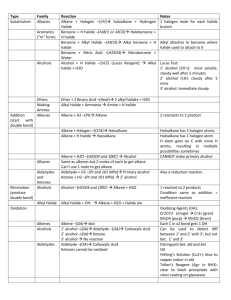
Substitution
An atom or group in the chain is replaced by another (2 reactants, 2 products)
1.
Alkanes
With: Halogens (for every halogen you want on the chain, you must use 1 mole of the diatomic)
Catalyst: UV light
Products: haloalkane and hydrogen halide
2.
Aromatics a.
With Halogens
Catalyst: FeBr3 or AlCl3
Products: halobenzene and hydrogen halide b.
With an Alkyl Halide (C-chain w/ a Halogen)
Catalyst: AlCl3
Products: alkyl benzene and hydrogen halide
This is how we get a halogen onto benzene c.
With Nitric Acid
Catalyst: H2SO4
Products: nitrobenzene and water
3.
Alcohols
With: Hydrogen Halide
Catalyst: ZnCl2 (Lucas Reagent)
Products: alkyl halide and water
Qualitative test for alcohols: rate of rxn differs for different alcohols due to the solubility of the resulting alkyl halides
1⁰ alcohols: most soluble, ppt formed >5 min
2⁰ alcohols: intermediate solubility, ppt formed ̴5 min
3⁰ alcohols: least soluble, ppts immediately
4.
Ethers
With: 2 binary acids (2 moles)
Catalyst: heat
Products: alkyl halides and water
5.
Ammonia (for preparing Amides)
With: alkyl halide
No catalyst
Products: amine and hydrogen halide
Addition
Adding groups or atoms to the chain by breaking a pi bond
1.
Alkenes a.
With Hydrogens (H-H)
Catalyst: platinum (Pt)
Product: alkane
Easiest way to make an alkane b.
With Halogens
Catalyst: CCl4
Product: haloalkane (2 halogen atoms, ex Cl-Cl) c.
With Hydrogen Halide*
Catalyst: NA
Product: haloalkane (1 hydrogen atom) d.
With Water*
Catalyst: H2SO4 + 100⁰C
Products: alcohol
*follow Markovnikov’s Rule where the H gets added to the C in the double bond that started with the most H’s.
1.
Alkynes
Same as Alkanes only 2 moles of each is needed, fully saturating the triple bond
2.
Aldehydes and Ketones
With: hydrogen (reduction!)
Catalyst: Pt and 101 MPa
Product: Aldehydes will make a 1⁰ alcohol (easiest way to do so), ketones will make a 2⁰ alcohol
Elimination
Removal of 2 atoms/groups to form a double bond
1.
Alcohols
Catalyst: H2SO4 and 100⁰C
Products: Alkene and water
2.
Alkyl Halides
With; hydroxide ion
Products: alkene + water + halide ion
Oxidation
loss of electrons by the C atom
oxidizing agents will usually result in a colour change (can used as qualitative tests)
Agents: a.
Cr2O7^2- : dichromate (orange) to chromium^3+ (green) to chromium
2+ (blue) [ogb] b.
MnO4^- : permanganate (purple) to manganese (V) oxide (brown) [pb]
1.
Alkenes
Oxidizing agent: MnO4^- or Cr2O7^2-
Product: “diol” (each C in the double bond gets an –OH group)
2.
Alcohols
Oxidizing agent: MnO4^- or Cr2O7^2-
Products: depends on type of alcohol
1⁰ alcohol: aldehyde carboxylic acid
2⁰ alcohol: ketone
3⁰ alcohol: NR ( no H on the C)
3.
Aldehydes
Product: carboxylic acid
Since ketones can’t be oxidized, oxidation rxns can be used as a qualitative test to distinguish between aldehyde and ketone
Oxidizing Agents:
MnO4^- : purple to brown in aldehyde, stays purple in ketone
Cr2O7^2- : purple to green in aldehyde, stays orange in ketone
Fehling’s solution (copper II solution): blue to an orangish brown precipitate in an aldehyde, stays blue in ketone
Tollen’s Reagent (silver ions in ammonia): clear and colourless to a black precipitate with a silver mirrored coating on the glassware in an aldehyde, stays colourless in a ketone
Known as the silver mirror test
Condensation
Liking 2 molecules by the removal of water
H comes from 1 molecule and OH comes from another
1.
Alcohols a.
With: another alcohol
Catalyst: H2SO4 + 140⁰C
Products: ether and water b.
With: carboxylic acid
Catalyst: H2SO4 + heat
Products: ester and water
2.
Amines
With: carboxylic acid
Catalyst: H2SO4 + heat
Products: amide + water
Hydrolysis
Splitting apart a molecule by adding water
1.
Esters: (reverse of a condensation rxn with alcohol and carboxylic acid)
2.
Amides: (reverse of a condensation rxn with amines)
CATALYSTS:
UV light
FeBr3
Alkane with Halogens (subs.)
Aromatics with Halogens (subs.)
AlCl3
H2SO4
Aromatics with Halogens (subs.)
Aromatics with Alkyl Halides (subs.)
Aromatics with HNO3 (subs.)
ZnCl2 (Lucas Reagent)
Alcohols with Hydrogen Halides (subs.)
∆
Ethers with 2 binary acids (subs.)
Pt
Pt + 101 MPa
CCl4
H2SO4 + 100⁰C
H2SO4 + 140⁰C
Alkene with Hydrogen (add.)
Alkyne with Hydrogen (add.)
Aldehyde or Ketone with Hydrogen (add.)
Alkene with Halogen (add.)
Alkyne with Halogen (add.)
Alkene with Water (add.)
Alkyne with Water (add.)
Elimination of Alcohols
Alcohol with alcohol (cond.)
H2SO4 + ∆
MnO4^-
Cr2O7^2-
Fehling’s Solution
Tollen’s Reagent
No catalysts
Alcohol with carboxylic acid (cond.)
Amine with carboxylic acid (cond.)
Alkanes, Alcohols and Aldehydes (ox.)
Alkanes, Alcohols and Aldehydes (ox.)
Aldehydes (ox.)
Aldehydes (ox.)
Alkene with Hydrogen Halide (add.)
Elimination of Alkyl Halides
Ammonia with Alkyl Halide (subs.)
Reaction Type
Combustion Complete H-C chain
Incomplete H-C chain
Substitution
(atom or group is replaced by another)
Addition
(adding groups or atoms to the chain by breaking a pi bond)
Elimination
(removal of a group or atom to form a double bond)
Oxidation
Participating
Group
Alkanes
Aromatics
Alcohols
Ethers
Ammonia
Alkenes
Alkynes
Aldehydes
Ketones
Alcohols
Condensation
(linking 2 molecules by the removal of water)
Alcohol
Amine
With Catalyst(s) Product(s)
O2
O2
Halogens
Halogens
Alkyl Halides
-
-
UV light
AlC3 or
FeBr3
AlCl3
CO2 + H2O
CO2 + H2O + CO + C
Haloalkene + Hydrogen
Halide
Halobenzene + Hydrogen
Halide
Alkylbenzene + hydrogen halide
Water + Nitrobenzene Nitric Acid
(HNO3)
H2SO4
Hydrogen Halide ZnCl2
(Lucas
Reagent)
2 binary acids Heat
Water + Alkyl Halide
2 Alkyl Halides + water
Amine + Hydrogen Halide Alkyl Halide -
Hydrogens Pt
Halogens CCl4
Hydrogen Halide -
Alkane
Haloalkane
Haloalkane
Water H2SO4 +
100⁰C
Alcohol
Same as Alkenes but with 2 moles of each
Hydrogen
-
-OH
Pt + 101
MPa
H2SO4 +
100⁰C
-
1⁰ Alcohol
2⁰ Alcohol
Water + Alkene
Water + Alkene + Halide Ion Alkyl Halides
Alkenes
Alcohol
Aldehyde
1⁰
2⁰
3⁰
MnO4^- or
Cr2O2^2-
Write
Oxidizing
Agent as catalyst
“diol” (each C in double bond gets an –OH)
Aldehyde Carboxylic Acid
Ketone
-
Carboxylic Acid MnO4^-
Cr2O7^2-
Fehling’s Solution
Tollen’s Reagent
Alcohol
Carboxylic Acid
Carboxylic Acid
H2SO4 +
140⁰C
Ether + Water
H2SO4 + ∆ Ester + Water
H2SO4 + ∆ Amide + Water
Qualitative Tests:
1.
Test for Type of Alcohol
Use Lucas Reagent (ZnCl2) in Substitution reaction of Alcohols with Hydrogen
Halide (products are water + haloalkane)
1⁰ alcohols are most soluble, therefore will precipitate in >5 minutes
2⁰ alcohols are intermediate, therefore will precipitate in ̴5 minutes
3⁰ alcohols are least soluble, therefore will precipitate immediately
2.
Test for Ketone or Aldehyde
Use one of 4 Oxidizing Agents:
I.
MnO4^1- : Ketone will stay purple, aldehyde will turn brown
II.
Cr2O7^2- : Ketone will stay orange, aldehyde will turn green
III.
Fehling’s solution (copper II solution): Ketone will stay blue, aldehyde will turn from blue to orange-brown precipitate (i.e. copper metal)
IV.
Tollen’s Reagent/Silver Mirror test (silver ions in ammonia): stays colourless in ketone, goes from colourless to a black precipitate with a silver mirrored coating on the glassware used with an aldehyde
Organic Compounds
5 characteristics:
1.
Made of Carbon chains or rings
2.
Covalent bonds
3.
Principle force: London Dispersion (all molecules will have it)
4.
Many isomers
5.
Properties are determined by functional groups of the molecules
Many organic compounds exist for 3 reasons:
1.
Carbon has 4 valence electrons therefore can form 4 bonds
2.
Carbon readily bonds with other Carbon atoms
3.
Carbon readily bonds with other atoms such as Oxygen, Nitrogen, Sulphur and Halogens
Molecular Forces
Intramolecular forces
1.
Exist within a molecule (covalent bonding)
2.
Broken down by chemical means to form new substances
3.
Determine the chemical properties of a substance (how they bond)
Intermolecular forces
1.
between 2 molecules
2.
much weaker than intramolecular forces therefore are easier to break
3.
physical changes (i.e. change of state) break or weaken these bonds but form no new substance (the molecule is still the same molecule)
4.
determine the physical properties of molecules and compounds (stronger forces = higher boiling point, melting point)
5.
3 types of intermolecular forces:
1.
London Dispersion
Based on the attraction of electrons to protons of another molecule
The more electrons or protons, the stronger the force (i.e. bigger molecules have a stronger LD force)
Everything has London dispersion
The weakest of the forces
2.
Dipole-Dipole forces
Based on oppositely charged ends due to an unequal distribution of charge on a molecule
Occur between polar molecules having dipoles
polarity determined by polarity of bond and shape of molecule
one side of the molecule will be slightly positive, the other side will be slightly negative
The stronger the polarity, the stronger the force
Will be soluble in other polar solvents, not water! (unless it has Hbonds)
3.
Hydrogen Bonding
A type of Dipole-Dipole force
Occur between Hydrogen in one molecule and highly electronegative atoms in another molecule (i.e. Fluorine, Oxygen or Nitrogen)
Strongest of the forces
Will be soluble in water
Isomers
Molecules with the same formula but different structure, 3 types:
1.
Structural: different arrangement of atoms, same organic family
2.
Functional: same formula, different organic families
3.
Geometric: different placement of groups around a double bond (“cis-trans” isomers)
Making Polymers
Addition Polymerization: alkene monomers joined through multiple addition reactions to form a polymer, reduces C=C to C-C
Condensation Polymerization: monomers are combined through multiple condensation reactions to form a polymer, water is produced (i.e. esterification)