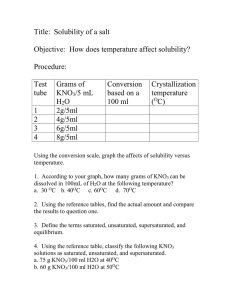
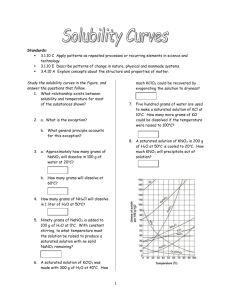
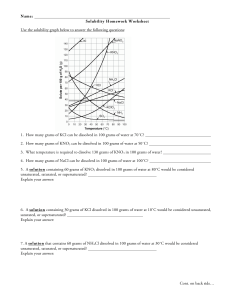
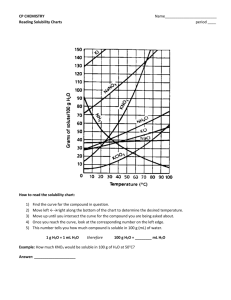
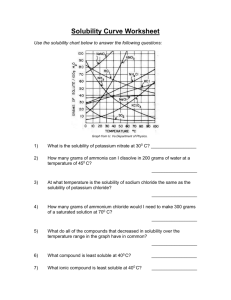
Homework Set 13-2: Solubility Curves
advertisement
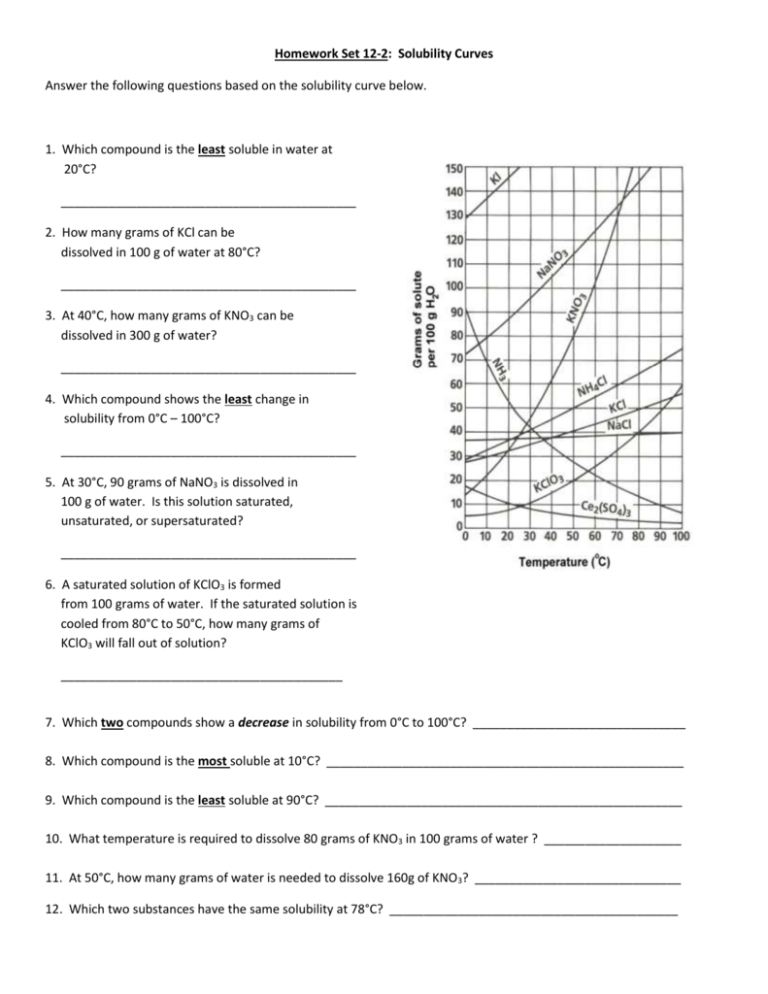
Homework Set 12-2: Solubility Curves Answer the following questions based on the solubility curve below. 1. Which compound is the least soluble in water at 20°C? ___________________________________________ 2. How many grams of KCl can be dissolved in 100 g of water at 80°C? ___________________________________________ 3. At 40°C, how many grams of KNO3 can be dissolved in 300 g of water? ___________________________________________ 4. Which compound shows the least change in solubility from 0°C – 100°C? ___________________________________________ 5. At 30°C, 90 grams of NaNO3 is dissolved in 100 g of water. Is this solution saturated, unsaturated, or supersaturated? ___________________________________________ 6. A saturated solution of KClO3 is formed from 100 grams of water. If the saturated solution is cooled from 80°C to 50°C, how many grams of KClO3 will fall out of solution? _________________________________________ 7. Which two compounds show a decrease in solubility from 0°C to 100°C? _______________________________ 8. Which compound is the most soluble at 10°C? ____________________________________________________ 9. Which compound is the least soluble at 90°C? ____________________________________________________ 10. What temperature is required to dissolve 80 grams of KNO3 in 100 grams of water ? ____________________ 11. At 50°C, how many grams of water is needed to dissolve 160g of KNO3? ______________________________ 12. Which two substances have the same solubility at 78°C? __________________________________________ 13. Determine if each of the following is unsaturated (U), saturated (S), or supersaturated (SS) (in 100 g of H2O). a. b. c. d. e. 55g of NH3 at 20°C 10g of Ce2(SO4)3 at 10°C 125g of KNO3 at 60°C 80g of NH4Cl at 50°C 12g of NH3 at 90°C _________ _________ _________ _________ _________ f. 70g of NaNO3 at 30°C g. 20g of KClO3 at 50°C h. 35g of NaCl at 100°C 14. Determine the solubility of each of the following. (in 100 g of H2O) a. b. c. d. KNO3 at 70°C NH4Cl at 90°C KI at 20°C KClO3 at 90°C ___________________ ___________________ ___________________ ___________________ _________ _________ _________