Name: Solubility Homework Worksheet Use the solubility graph
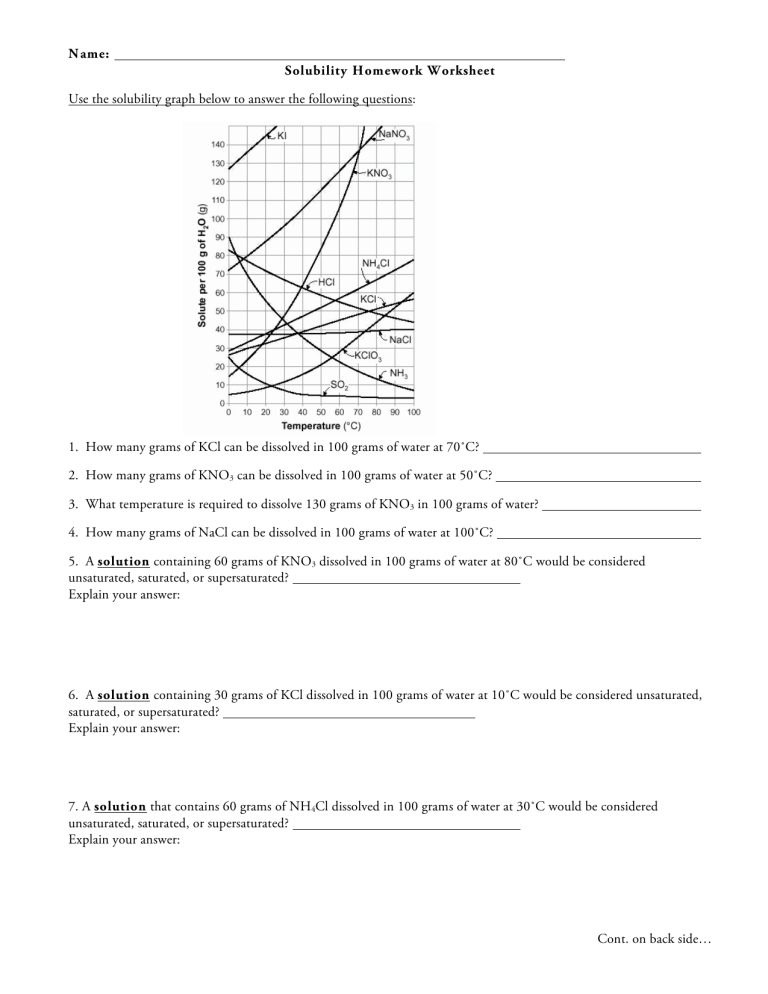
N ame:
Solubility Homework W orksheet
Use the solubility graph below to answer the following questions:
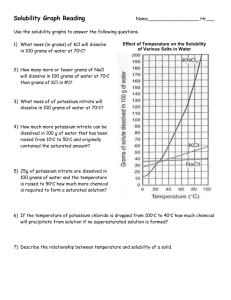
1. How many grams of KCl can be dissolved in 100 grams of water at 70˚C?
2. How many grams of KNO
3
can be dissolved in 100 grams of water at 50˚C?
3. What temperature is required to dissolve 130 grams of KNO
3
in 100 grams of water?
4. How many grams of NaCl can be dissolved in 100 grams of water at 100˚C?
5. A solution containing 60 grams of KNO
3
dissolved in 100 grams of water at 80˚C would be considered unsaturated, saturated, or supersaturated?
Explain your answer:
6. A solution containing 30 grams of KCl dissolved in 100 grams of water at 10˚C would be considered unsaturated, saturated, or supersaturated?
Explain your answer:
7. A solution that contains 60 grams of NH
4
Cl dissolved in 100 grams of water at 30˚C would be considered unsaturated, saturated, or supersaturated?
Explain your answer:
Cont. on back side…
8. You dissolve 10 g KClO
3
in 100 g water at 40 o C. Is this solution unsaturated, saturated, or supersaturated?
How much more KClO
3
must be added to form a saturated solution at 40 o C?
9. What is the minimum mass of water needed to dissolve 10 g KClO
3
at 40 o C? Show your work:
10. A supersaturated solution of KNO
3
is formed by adding 90 g KNO
3
to 100 g water at 25 o C, heating until the solute completely dissolves, and then cooling the solution to 45 o C. If the solution is agitated, how much KNO
3
will precipitate?
11. How much 45 o C water would have to be added (to the original 100 g water in problem #10) to just dissolve all the
KNO
3
that precipitated? Show your work:
12. How much NaNO
3
will dissolve in 145 g water at 30 o C? Show your work: