Solubility Curves worksheet all pages 1 thru 4
advertisement
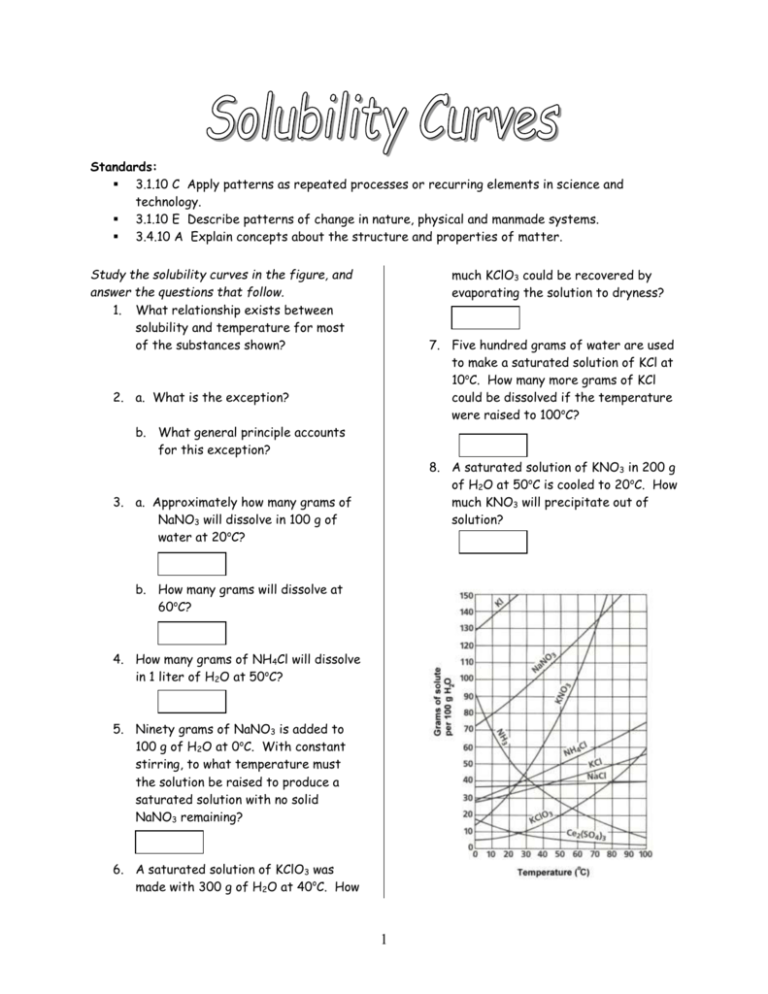
Standards: 3.1.10 C Apply patterns as repeated processes or recurring elements in science and technology. 3.1.10 E Describe patterns of change in nature, physical and manmade systems. 3.4.10 A Explain concepts about the structure and properties of matter. Study the solubility curves in the figure, and answer the questions that follow. 1. What relationship exists between solubility and temperature for most of the substances shown? much KClO3 could be recovered by evaporating the solution to dryness? 7. Five hundred grams of water are used to make a saturated solution of KCl at 10oC. How many more grams of KCl could be dissolved if the temperature were raised to 100oC? 2. a. What is the exception? b. What general principle accounts for this exception? 8. A saturated solution of KNO3 in 200 g of H2O at 50oC is cooled to 20oC. How much KNO3 will precipitate out of solution? 3. a. Approximately how many grams of NaNO3 will dissolve in 100 g of water at 20oC? b. How many grams will dissolve at 60oC? 4. How many grams of NH4Cl will dissolve in 1 liter of H2O at 50oC? 5. Ninety grams of NaNO3 is added to 100 g of H2O at 0oC. With constant stirring, to what temperature must the solution be raised to produce a saturated solution with no solid NaNO3 remaining? 6. A saturated solution of KClO3 was made with 300 g of H2O at 40oC. How 1 Solubility Curves … Part II 1. 7. At what temperature do KBr and KNO3 have the same solubility? What is that solubility? Temperature: Solubility: What is plotted on … 8. At what temperature does NaClO3 have a solubility of 180 g/100 g H2O? The X axis: The Y axis: 2. In general what does the graph illustrate? 9. At what temperature does KNO3 have a solubility of 45 g/100 g H2O? 3. How would you describe what happens to the solubilities of these substances as temperature increases? 10. What general statement can you make about the solubility of NaCl with increasing temperature compared with those of KBr, KClO3, and NaClO3? 4. What is the solubility of each of the four materials at 0oC? a. NaClO3 b. KBr c. KNO3 d. NaCl 5. Which substance is most soluble and which is least soluble at 0oC? Most: Least: 6. At 80oC, which substance is most soluble and which is least soluble? Most: Least: 2 Solubilities of Two Compounds 5. Calculate the molarity of a saturated solution of substance A at 20oC. (Assume for simplicity that the solubility per 100 g of water is equivalent to the solubility per 100 mL of solution.) 1. The diagram to the right shows how the solubilities of two compounds, A and B, change with temperature. One of the substances is lead (II) chloride (PbCl2) and the other is carbon dioxide (CO2). Which curve do you think belongs to each substance? A= 6. Calculate the molarity of a saturated solution of substance B at 20oC. B= 2. What is the basis for your answer to the above question? 3. What are the solubilities of A and B at 0oC? A= B= 4. What are the solubilities of A and B at 20oC? A= B= 3 Stock Solutions A group of students made a number of solutions of known concentrations for the class stockroom. Unfortunately, they neglected to record all the information regarding the way in which the solutions were made. From the information provided in the chart below, determine the ten missing values and write them on the lines. Solute formula Solute Mass Solution Volume KOH 7.80 g 500. mL LiCl ______ CaCl2 9.00 g Al2(SO4)3 12.3 g K3PO4 ______ 4.00 L 250. mL ______ 250. mL Molarity ______ 0.125 M Solute formula Solute Mass KClO3 122.5 g NH4Br HNO3 ______ 0.900 M HCl 0.324 M (NH4)2SO4 4 ______ 20.0 g ______ 44.2 g Solution Volume ______ 2.00 L 500. mL 750. mL 600. mL Molarity 1.00 M 0.50 M ______ 0.044 M ______






