Mesostructured Germanium with Cubic Pore Symmetry
advertisement

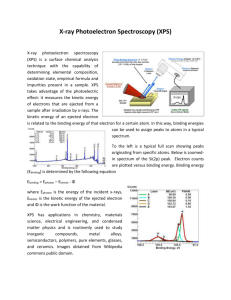
Mesostructured Germanium with Cubic Pore Symmetry Gerasimos S. Armatas and Mercouri G. Kanatzidis* Department of Chemistry, Michigan State University, East Lansing, MI 48824, U.S.A. Supplementary Notes Pair distribution function (PDF) on MSU-Ge-1 after partial oxidation Figure S1. Pair distribution function (PDF) on MSU-Ge-1 material obtained after partial oxidation (3d). The Peaks at 1.8, 2.9 and 4.2 Å are interatomic correlations from Ge-O bonds, Ge···Ge second neighbors (via Ge-O-Ge moieties) and Ge···Ge third neighbors. The absence of these peaks in the PDF of pristine MSU-Ge-1 itself (Figure 3A) confirms that Ge-O-Ge moieties are not present. Powder X-ray diffraction pattern of air oxidized MSU-Ge-1 Figure S2. X-ray diffraction patterns of an MSU-Ge-1 sample taken at different times from being fresh (as-prepared) up to one month later as it were continuously exposed to air. The partial oxidation of this sample gives rise to a sub-oxide framework as indicated by the blue shift in the energy gap (Figure 3c). The X-ray pattern does not change significantly which suggests that this solid state reaction maintains the overall cubic symmetry and gyroid minimal surface of the inorganic network as oxidation proceeds, thereby being a topotactic process. X-ray photoelectron spectroscopy (XPS) To ensure that we did not have GeO2 and nanocrystalline Ge in MSU-Ge-1, we perform X-ray photoelectron spectroscopy (XPS) experiments. A Perkin Elmer Phi 5400 ESCA system was used for experimentation with a Magnesium K X-ray source. Samples were analyzed at pressures between 10-9 and 10-8 torr with a pass energy of 29.35 eV and a take-off angle of 45°. Atomic concentrations were determined using previously determined sensitivity factors. All peaks were referenced to the signature C1s peak for adventitious carbon at 284.6 eV. 31.7 31.5 MSU-Ge-1(b) Photoelectron Intensity (a.u.) Photoelectron Intensity (a.u.) MSU-Ge-1(a) 32.8 30.6 26 28 30 32 34 36 38 26 40 31.4 29.3 28 30 30.1 30 32 36 32.9 Ge-metal 32.9 28 34 38 40 GeO2 Photoelectron Intensity (a.u.) Photoelectron Intensity (a.u.) 29.2 26 32 Binding Energy, eV Binding Energy, eV 34 Binding Energy, eV 36 38 40 26 28 30 32 34 36 38 40 Binding Energy, eV Figure S3. XPS data on two different samples of MSU-Ge-1, GeO2 and crystalline Ge. The peak at 32.9 eV in the spectrum of crystalline Ge is due to GeO2 impurity phase. We observed 3d core level Ge spectra which show that the binding energy peak occurs at a unique place at 31.5-31.7 eV. This value is different from those of crystalline Ge (29.2 eV) and in GeO2 (32.9 eV) respectively. This confirms that the MSU-Ge-1 material is not composed of a combination of nanocrystals of Ge and GeO2. Rather MSU-Ge-1 is a novel material. We are reluctant to try to extract oxidation states from these data because we do not know the precise Ge geometric environment and the fact that most Ge atoms in the gyroid mesostructured continuous Ge framework lie on the surface. There are no model compounds known in the literature to make further comparisons with XPS spectra.