EMS and Bohr Model of Hydrogen
advertisement

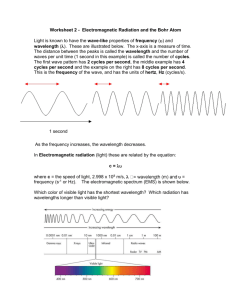
Electromagnetic Spectrum and the Bohr Model of Hydrogen Name: __________________ I. Electromagnetic Spectrum 1. List the 7 types of radiation that make up the electromagnetic spectrum. 2. Of the types of radiation, which has the longest wavelength? 3. Identify the type of radiation for each wavelength given: a. 1 x 10-5 m _____________________________ b. 1 x 10-13 m _____________________________ c. 1 x 102 m _____________________________ 4. Identify the color of visible light radiation for each wavelength given: a. 6.8 x 10-7 m ____________________________ b. 5.2 x 10-7 m ____________________________ 5. Given that the wavelength and frequency of radiation are inversely proportional, identify the type of radiation with the greatest frequency? 6. Given that frequency and energy are directly proportional to each other, identify the type of radiation with the lowest energy? II. Bohr Model for Hydrogen Atom 7. Which energy level (i.e. n= ?), represents the ground state? (the energy level closest to the nucleus) 8. Does an electron absorb or release energy as it transitions from n=2 to n=6? 9. Does an electron absorb or release energy as it transitions from n=3 to n=1? 10. Fill in the chart with the missing information? ninitial nfinal 6 3 4 1 wavelength (nm) 486 2 1 1282 11. What color of light is associated with the following energy level transitions? a. n=6 to n=2 ____________________________ b. n=3 to n=2 ____________________________ Type of radiation Reading Guide Chapter 4 Section 1 (NCCS: 1.1.2-1.1.3) Name: __________________________ 1. Define the following terms: Electromagnetic radiation Electromagnetic spectrum Wavelength Frequency Quantum Photon Ground State Excited State Line Emission spectrum 2. a. b. c. Referring to the diagram above: which wave has the longest wavelength? which wave has the highest frequency? which wave has the greatest energy? 3. Using Fig. 4-5 draw a colored model of the line emission spectrum of hydrogen, labeling the specific wavelengths associated with each spectral line. 4. Referring to page 96, “Bohr Model of the Hydrogen Atom” paragraph three, complete the following: While in a specific orbit the electron of hydrogen can neither gain nor lose energy. It can however, move to a higher energy orbit by __________________ an amount of energy equal to the difference in energy between the highest energy orbit and the ___________________________________________. When a hydrogen atom is an ____________________ state, its electron is in a higher energy orbit. When the atom “ falls back” from the excited state, the electron drops down to a ______________ ______________________ orbit. In the process, a ______________ is emitted that has an energy equal to the difference between the initial high energy orbit and the final low energy orbit. “This creates the hydrogen line emission spectrum that we observe... teacher input”.