electromagnetic spectrum and flame tests
advertisement

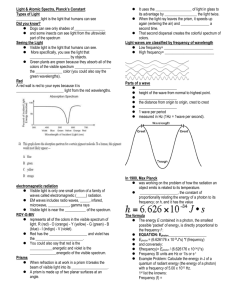
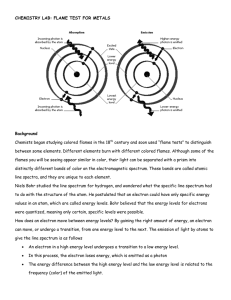
ELECTROMAGNETIC SPECTRUM AND FLAME TESTS Agenda • • • • Day 13 - Flame Test Lesson: Lesson: PPT, Handouts: 1.PPT Handout; Text: 1. P. 20; 439-440- Qualitative Analysis Involving Colours • HW: 1. P.441 # 5,6,9; 2. Finish all the worksheets Flame Test Demo- Pg. 439 Compound Lithium chloride Potassium chloride Copper (II) chloride Cation Li+ K+ Cu2+ Sodium chloride Strontium chloride Na+ Sr2+ Colour • • • • • • • • • • • • • • • • • • • • • • • • • • • Flame Test Colors Symbol Element As Arsenic B Boron Ba Barium Ca Calcium Cs Cesium Cu(I) Copper(I) Cu(II) Copper(II) Cu(II) Copper(II) Fe Iron In Indium K Potassium Li Lithium Mg Magnesium Mn(II) Manganese(II) Mo Molybdenum Na Sodium P Phosphorus Pb Lead Rb Rubidium Sb Antimony Se Selenium Sr Strontium Te Tellurium Tl Thallium Zn Zinc Color Blue Bright green Pale/Yellowish Green Orange to red Blue Blue non-halide Green halide Blue-green Gold Blue Lilac to red Magenta to carmine Bright white Yellowish green Yellowish green Intense yellow Pale bluish green Blue Red to purple-red Pale green Azure blue Crimson Pale green Pure green Bluish green to whitish green Let’s Review Energy – Radiation of different wavelengths affect matter differently – certain wavelengths (near infrared) may burn your skin with a heat burn, overexposure to X radiation causes tissue damage. These diverse effects are due to differences in the energy of the radiation. Radiation of high frequency and short wavelength are more energetic than radiation of lower frequency and longer wavelength. While light exhibits many wavelike properties, it can also be thought of as a stream of particles. Each particle of light carries a quantum of energy. Einstein called these particles PHOTONS. A photon is a particle of electromagnetic radiation having zero mass and carrying a quantum of energy. Albert Einstein • The electromagnetic spectrum is continuous showing no breaks between different energy waves. • When looking at visible, ‘white light’ through a spectroscope there is a continuous spectrum of coloured light – R O Y G B V (the colours of the rainbow). • Red has the lowest energy with the largest wavelength, smallest frequency and violet has the greatest , with lower wavelength and greater frequency. The Hydrogen-Atom Line-Emission Spectrum When investigators passed an electric current through a vacuum tube containing hydrogen gas at low pressure, they observed the emission of a characteristic pinkish glow. When a narrow beam of the emitted light was shined through a prism, it was separated into a series of specific frequencies (and therefore specific wavelengths, c =) of visible light. The bands of light were part of what is known as hydrogen’s LINEEMISSION SPECTRUM. When gaseous atoms of elements are heated and the electrons absorb sufficient energy to ‘escape’ their ground state configuration, ‘excited’ electrons jump to higher energy levels ( the greater the distance between the nucleus of the atom and the energy level the greater the energy needed by the electron to travel to that level). ‘Excited electrons’ are unstable and so return to ground state releasing the absorbed energy in discrete energy emissions patterns. In the visible spectrum, these emissions appear as discrete lines of colour in a spectroscope. Bohr’s Theory of the Atom: Bohr’s Model of the Atom The hydrogen atom emits visible light when its electron moves from the third through sixth energy levels to the second energy level. Ultraviolet radiation is emitted when the electron moves from the second through sixth energy levels to the first energy level. Infrared radiation is emitted when the electron moves from the fourth through sixth energy levels to the third energy level. Complete the following diagram so the purple and red arrows were used to represent the transitions that result in ultraviolet and infrared emissions to the diagrams below. FLAME TESTS Flame tests rely on the idea that each element has a characteristic set of emissions and will produce a unique and characteristic colour when heated. The sample is identified by comparing the observed flame color against known values from a table or chart. Q: “ Spectra lines are the fingerprints of elements”. Explain what is meant by this statement. Q: According to the Bohr theory, what happens to an electron in an atom as it absorbs energy and as it releases energy?