Objective Homework - Mr. Smith`s Pre-AP Chemistry
advertisement

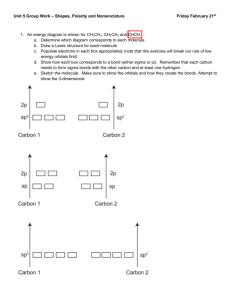
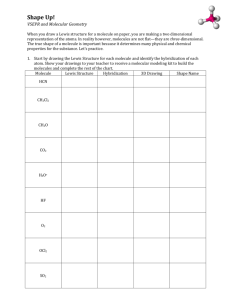
Pre-AP Chemistry – Unit 5, Chemical Bonding and Naming Molecular Compounds Objective 1: Students will describe the sharing of electrons to form covalent bonds, name the molecules, and use Lewis structures to model the resulting formulas. [B.9.C.1, B.9.C.2, P.5.C.2] Objective 2: Students will use VSEPR theory and molecular orbital theory to predict and explain the shapes of molecules. [B.9.C.3] Objective 3: Students will compare and contrast various interatomic and intermolecular forces. [B.9.C.4, B.10.C.1] Objective 1 1. Draw Lewis structures for the following compounds: SO2, CO2, H2O. 2. Draw a Lewis structure for ClF5 and explain how this violates the octet rule. More than the octet rule; period 3 or beyond can have more than 8 electrons 3. Draw a Lewis structure for BF3 and explain how it violates the octet rule. Boron can have less than the octet rule; usually 6 electrons 4. Draw a Lewis structure for NO2 and explain how it violates the octet rule. Why is this molecule very unstable? What is the special name for a molecule like this? This molecule has an odd number of valence electrons and is an exception to the octet rule. This molecule is very reactive because of the lone electron. This is called a free radical. We did not discuss this in class and you will not be held responsible for that. 5. Draw the resonance form of SO3. 6. Name the following molecules: N2O5 dinitrogen pentoxide P4O10 tetraphosphorus decoxide SO3 sulfur trioxide SiH4 silicon tetrahydride NH3 ammonia 7. Write formulas for the following: nitrogen trifluoride NF3 diarsenic trioxide As2O3 bromine pentaiodide BrI5 xenon tetrafluoride XeF4 krypton dioxide KrO2 8. List some common properties of molecular compounds. How do these differ from ionic compounds? Molecular compounds have lower melting and boiling points and are often liquids or gases. Ionic compounds have high melting and boiling points and are crystalline solids. Objective 2 1. Define VSEPR and explain how it relates to molecular shape. Valence Shell Electron Pair Repulsion- predicts shape of molecules based on the theory that electrons are trying to be as far away from each other as possible. 2. Describe the bonding in sigma and pi bonds. Which is stronger in and of itself? Why? Sigma bond- when two atomic orbitals combine to form the molecular orbital that is symmetrical along the axis connecting the nuclei Pi bond- the bonding electrons are likely to be found above and below the bond axis (weaker than sigma) 3. What is the reasoning behind the theory of orbital hybridization? Explain using the methane molecule. Hybridization Theory states that "a covalent bond is formed by the overlap of two singly occupied hybrid or atomic orbitals. Hybrid atomic orbitals are created by mixing together atomic orbitals to form an equal number of new hybrid atomic orbitals" this increases the stability of the molecule. Carbon in methane will promote one of its s electrons to a p electron; making the hybridization sp3 4. For the following molecules/ions provide the (a) Lewis structure, (b) the number of bonding domains, (c) the number of nonbonding domains, (d) number of bonds, (e) number of bonds, (f) hybridization of the central atom, and (g) the molecular geometry: SF4 b) 4 c) 2 d) 4 e) 0 f) sp3d g) seesaw CO32- b) 3 c) 0 d) 3 e) 1 f) sp2 g) trigonal planar BH3 b) 3 c) 0 d) 3 e) 0 f) sp2 g) trigonal planar NO b) 1 c) 1.5 d) 1 e) 1 f) don’t worry about it g) linear PCl5 b) 5 c) 0 d) 5 e) 0 f) sp3d g) trigonal bipyramidal Objective 3 1. Define the following: van der Waals forces- two weakest attractions between molecules are collectively know as van der dipole interactions- when polar molecules are attracted to one another dispersion forces- weakest of all molecular interactions; caused by the moving of electrons hydrogen bonding- attractive force in which hydrogen covalently bonded to a very electronegative atom is also weakly bonded to an unshared electron pair of another electronegative atom 2. Water and methane have similar masses and are composed of elements that are in a similar location on the periodic table. Explain why methane exists as a gas at room temperature while water exists as a liquid. Methane is a nonpolar molecule and has dispersion forces which are very weak. Therefore it can very easily become a gas. Water is a polar molecule that experiences hydrogen bonding (strong). Therefore it would take a lot of energy to make it a gas. 3. Explain how dispersion forces form in molecules with no polarity, such as CO2. When molecule come close to each, electrons shift inside the atom; creating a brief dipole. 4. Use a diagram to show what happens when HF is placed between charged plates, including how the HF molecules are attracted to each other. When polar molecules are placed between oppositely charged plates, they tend to become oriented with respect to the positive and negative plates. ClBr3 CO3 -2 see below Lewis Structure 3 3 2 0 3 3 0 1 sp3d sp2 T-shaped trigonal planar Number of Bonding Domains Number of Non-Bonding Domains Number of Sigma Bonds Number of Pi Bonds Hybridization of Central Atom Molecular Geometry