Lesson Plan
advertisement

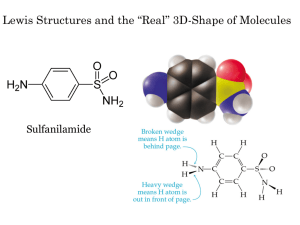
Physical Sciences: MOVING INTO 3-D. Grade Level & Duration: 11th Grade. One 50 Minute Class Period Prepared by: Fee Mtshiya Subject: Moving Into 3-D. Using Valence Shell Electron Pair Repulsion Theory to go from 2 to 3-Dimensional understanding of Molecules. Overview & Purpose (STEMcinnati theme) Education Standards Addressed What will be learned and why it is useful: What state/county education standards that this lesson satisfies. The State of Ohio. Hamilton County ~ Cincinnati Public Schools. The goal is to learn how to use the Valence Shell Electron Pair Repulsion (VSEPR) theory to be able to predict the shape of molecules. The students draw on what they have learnt about the periodic table, valence electrons, Lewis Electron-Dot structures, single and multiple bonds and apply this knowledge to the VSEPR theory to be able to predict molecular shapes. The essence is ‘Going from a 2 to 3-Dimensional’ appreciation of molecules. Probably the most common Misconception students have is that molecules and compounds exist in nature in the 2-Dimensional form that is seen in textbooks and this lesson directly addresses that misconception. Applications to the real world: The last activity in this lesson is called ‘Cement Soup’ and is based on how Structural Engineers use plasticizers when working with cement. The engineers have to have a knowledge of how these molecules function based on their 3-D structures which ultimately dictates their chemical and physical properties. Societal impact: Chemistry deals with the composition and properties of matter that we use in day to day and therefore there is a direct connection between the subject matter and society – the houses we live in, food we eat, transportation we use etc. Career connections: It is important for not only engineers and scientists to understand the nature of properties (such as structural engineers and cement in the demo) but to be effective informed workers, the construction workers and workers in the cement producing factories also need to know how to deal with their materials. Knowing the characteristics and potential hazards of substances makes sense in any career. Specific State of OHIO Science Academic Education Standards Addressed. Physical Sciences; 11-12 program: Explain how variations in the arrangement and motion of atoms and molecules form the basis of a variety of biological, chemical and physical phenomena. Scientific Inquiry; 11-12 program: Make appropriate choices when designing and participating in scientific investigations by using cognitive and manipulative skills when collecting data and formulating conclusions from the data. Select Goals and Objectives Teacher Guide Goals and Objectives (Specify skills/information that will be learned.) The Goal or Overall Aching Idea is to have the students Learn/Appreciate how to use the Valence Shell Electron Pair Repulsion (VSEPR) theory to model and predict the shape of a molecule by analyzing the Lewis-Electron Dot Structure of the molecule. By the end of the lesson the students should be able to Apply their knowledge of VSEPR to predict molecular shapes. Recognize how Lewis-Electron Dot Structures for elements and compounds are derived. The students should be able to not only recall how to come up with these structures but should also be comfortable explaining the process using appropriate scientific terminology such as valence electrons, octet, octet rule, covalent bond, ionic bond lone pairs, resonance structures, single, double and triple bonds. Terms in Bold are under ‘Knowledge’ in Bloom’s Taxonomy. Explain how the VSEPR model can be used to predict molecular shape based on LewisElectron Dot Structures and an understanding of how electrons involved in the different types of bonds interact with each other. Be prepared to discuss molecular shapes such as linear, bent, trigonal planar and tetrahedral can be explained in terms of bonds and repulsion between electrons. Illustrate the molecular shapes using examples. Terms in Bold are under ‘Comprehension’ and ‘Application’ in Bloom’s Taxonomy. Select Instructional Strategies – Information (Give and/or demonstrate necessary information) Materials Needed Stationery Bag of multi colored pipe-cleaners 2 tomatoes: 1 ripe and the other not yet ripe Tight seal container to store tomatoes 3 medium sized buckets Small bag/container of Cement Water A small vial of Polycarboxylate Utilize Technology Power point presentation, creative fly ins Interactive Discussion and Group Activities The lesson starts out as an interactive teacher-guided discussion (to lay out the basic information students will draw on) that will lead into group activities that will encourage inquiry and discovery learning. Catch A look at The Hormone Ethene aka Ethylene using Tomatoes and Pipe Cleaners (approx 5 minutes) The teacher walks around the class juggling/showing the students the two tomatoes (one ripe and the other ripening) and asking what the difference is. Probing questions and suggestions from the instructor lead to the fact that the ripe tomato has more of the hormone ethene (ethylene) than the one that is ripening. The teacher then shows the students pipe cleaners of three different colors twisted into different shapes. A large circular shape represents carbon; a smaller circular shape of a different color represents hydrogen and small little C-shape hook structures of a different color represent electrons. The teacher puts the shapes together as he/she asks the students what each shape represents with regards to ethene. The catch ends with a reiteration of the fact that C2H4 is the molecular formula for ethene, the hormone tomatoes release as they ripen. Other Resources (e.g. Web, books, etc.) Additional Notes Require Learner Participation In Class Pre-Assessment Quiz; (approx 5 minutes) Activity (Describe the independent activity to reinforce this lesson) Questions handed out by instructor with the help of students. Students work individually and answer questions to the best of their ability. One word answers. Fellow student volunteer collects papers once quiz is completed. PRE-ASSESMENT Questions: 1) In the demonstration we just we had 4 electrons shared between the two Carbon atoms; what would we call such a bond? 2) There is a name for the outermost electrons that we work with when we create bonds between atoms. What is that name? 3) Is it True or False to say that Hydrogen doesn’t have to fulfill the octet rule? The atoms of the other elements have to have 8 electrons in their outermost orbital whereby Hydrogen needs 2 electrons to be fulfilled. 4) Fill in the Blank. The ___ of a molecule is determined by the valence electrons surrounding the central atom. 5) There is a theory that can be used to predict the shape of a molecule. We need to understand Lewis Structures to apply it. The theory is abbreviated to 5 letters; they are: _ _ _ _ _ Interactive Group-based Discovery Learning Activity; (approx 20 minutes); The pipe cleaner demonstration in the catch provides the foundation for the class activity. The students are divided into groups and for each type of molecular structure, the teacher calls on 2 students from each group to come up to the board:~ 1 draws the Lewis Structure of a particular molecule on the board (the student is free to ask for help from the group) and once this student is done the 2 nd comes up and uses circular pipe cleaners of different colors to represent the molecule 3-Dimensionally (similar to what the teacher did in the catch). The Student of course would not be expected to know the structure right away so as he/she looks at the Lewis Structure the other group member has drawn and tries to assemble the circles, the teacher explains how the electrons in the molecule are spaced, how they interact, what the molecular shape is called, what the bond angles are and any other important points to remember about that particular shape. Once the student is done, a power point slide of the correct molecular shape is shown to the class (thus the students either celebrate that they got it right or not!) and another slide is shown with other examples of molecules with that molecular shape. Here is an example of how the scene would play out when the teacher is presenting the molecular structure of ‘Tetrahedral’ to the class: The teacher calls on someone from Group 1 to come up to the board and draw the Lewis Structure for methane, CH4. The group decides on Jack who with some input from his group members draws it on the board. It is correct and the group is commended. The teacher then calls on another volunteer to come and assemble it on the board using Jack’s drawing as a guide. Jill comes up sees that Carbon has 4 Hydrogen atoms surrounding it and they are more or less evenly spaced apart. As Jill manipulates the structure, the teacher shows the class the tetrahedron shape and tells them that this molecule is going to have a tetrahedral shape with bond angles of 109.5 and no polarity in the molecule. With all this information Jill may or may not get the shape right. Her time is up when the teacher is done explaining. The teacher holds up her model as she pulls up the correct 3D rotating structure of methane on her power point. Again the group either celebrates that they got it right or not and the teacher takes this opportunity to correct mistakes and address common misconceptions that the students may have. The next slide the teacher will show will have rotating 3-D structures of NH4+, SO42-, PO43-, and Ni as further examples of the tetrahedral structure. These will not be labeled and students (in other groups) are asked to name them so they begin to recognize them as common molecules they will work with in chemistry. The process is repeated for the molecular structures of: Linear, Trigonal Planar & Trigonal Pyramid. Trigonal Bi-pyramidal and Octahedral structures can be covered at the teacher’s discretion. Group scores can then be tallied and reward(s) such as extra credit could be given to the group that not only got the right Lewis Structures and Molecular Structures but also worked quickly and communicated well. “Cement Soup” Mini Experiment ~ Bent (Angular) Molecular Structure (approx 6 minutes) The teacher draws the Lewis Structure of Water on the board and proceeds to assemble the molecular structure him/herself and shows the classroom. A power point slide with Water and other molecules with bent (angular) structures such as sulfur dioxide, ozone and nitrogen dioxide is pulled up. The teacher explains that the reason for looking at this structure differently is because there is an accompanying experiment. The experiment gives students the real life application of the subject matter in engineering. The molecular structure of water leads to a certain physical property: polarity. The polarity of water is something that has real life engineering implications that engineers (structural engineers in this case) have to address in their work. The teacher calls on a volunteer, someone who has not come up yet. The volunteer demonstrates the polarity of water and how it can be overcome in a mixture of cement and water. The quick demonstration consists of a small bucket containing a mixture of cement and water. As the volunteer stirs the mixture (tilting the bucket so everyone sees), it is pointed out that this clumpy material is very hard to work with out on the field as it does not flow easy. A PowerPoint slide is pulled up showing the mechanism of opposite charges attracting each other and forming clumps or flocs. A few scoops of the mixture are placed into another bucket and approximately 5ml of the plasticizer polycarboxylate is added. It is explained that a plasticizer is substance that increases the fluidity of the material it’s added to. As the student then stirs this mixture it is evident that this mixture is much more fluid. A final PowerPoint slide is pulled up showing ‘like’ charges repelling each other and the material/substance doesn’t have as many clumps if any at all. The fluidized mixture can be poured into a third bucket for an enhanced visual effect. This mini-experiment concludes the lesson. The Post-Assessment and the Review (Essential Questions) are next. Evaluate (Assessment) (Steps to check for student understanding) In Class Post-Assessment Quiz; (approx 5 minutes) Questions handed out by instructor with the help of students. Students work individually and answer questions to the best of their ability. One word answers. Teacher collects papers once quiz is completed. The Post-Assessment is the same as the Pre-Assessment. REVIEW ~ Essential Questions: (approx 5 minutes) What is the VSEPR theory exactly and why is it useful? It is a model that allows us to predict the shapes of molecules by using the Lewis Structures of those molecules. The VSEPR theory states something very important about electron pairs in the valence shells of atoms. What does it state? Valence Shell Electron pairs will stay as far apart as possible so that the repulsions between them are minimized. What are the molecular shapes predicted by the VSEPR theory? Linear, Bent, Trigonal, Planar, Tetrahedral & Trigonal Pyramid. Approximate lesson Time: 44 minutes Allowance for Classroom Management: 6 minutes