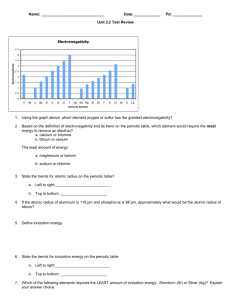
Honors Chemistry Unit 2 Study Guide Label all of the parts of the
advertisement
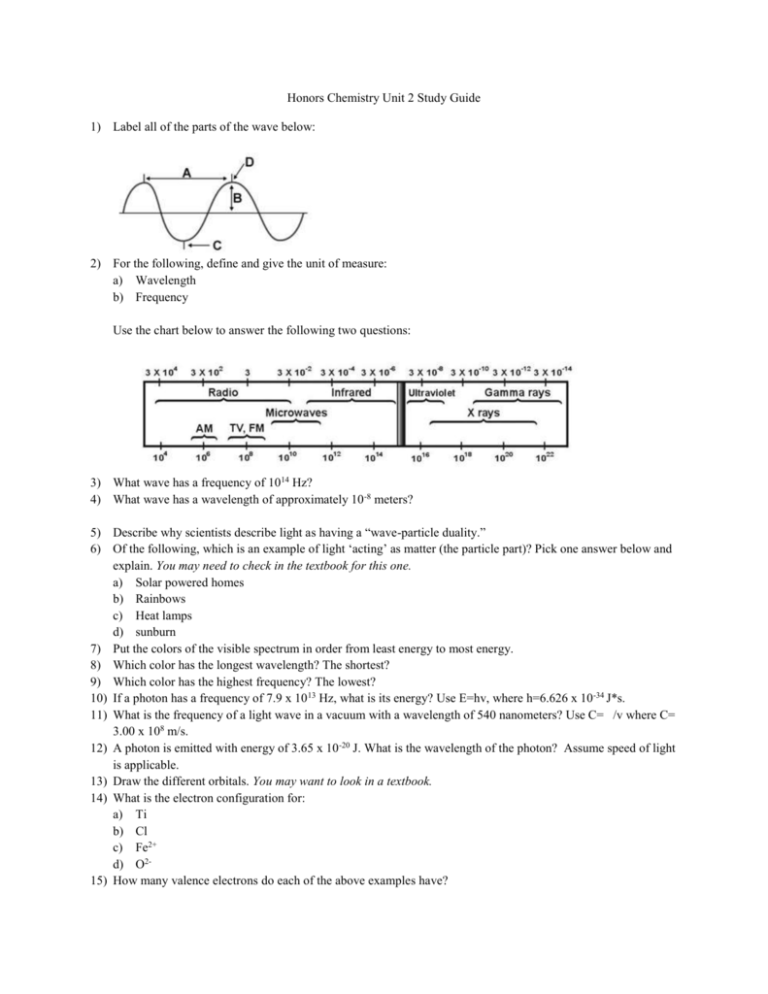
Honors Chemistry Unit 2 Study Guide 1) Label all of the parts of the wave below: 2) For the following, define and give the unit of measure: a) Wavelength b) Frequency Use the chart below to answer the following two questions: 3) What wave has a frequency of 1014 Hz? 4) What wave has a wavelength of approximately 10-8 meters? 5) Describe why scientists describe light as having a “wave-particle duality.” 6) Of the following, which is an example of light ‘acting’ as matter (the particle part)? Pick one answer below and explain. You may need to check in the textbook for this one. a) Solar powered homes b) Rainbows c) Heat lamps d) sunburn 7) Put the colors of the visible spectrum in order from least energy to most energy. 8) Which color has the longest wavelength? The shortest? 9) Which color has the highest frequency? The lowest? 10) If a photon has a frequency of 7.9 x 10 13 Hz, what is its energy? Use E=hv, where h=6.626 x 10 -34 J*s. 11) What is the frequency of a light wave in a vacuum with a wavelength of 540 nanometers? Use C= /v where C= 3.00 x 108 m/s. 12) A photon is emitted with energy of 3.65 x 10 -20 J. What is the wavelength of the photon? Assume speed of light is applicable. 13) Draw the different orbitals. You may want to look in a textbook. 14) What is the electron configuration for: a) Ti b) Cl c) Fe2+ d) O215) How many valence electrons do each of the above examples have? 16) Draw the electron dot structure for bromine. 17) Label the blocks on the periodic table below: 18) In the figure below, label the names and valence electrons of each group (you may want to check in a textbook): 19) As you move across the periodic table from A) left to right, and B) top to bottom, what happens to the following trends? a) Electron affinity b) Electronegativity c) Ionization energy d) Atomic radius 20) Give definitions for the following: a) Electron affinity b) Electronegativity c) Ionization energy d) Atomic radius e) Metal (also give an example and one property) f) Nonmetal (also give an example and one property) g) Metalloid (also give an example and one property) 21) Put the following in order of increasing atomic/ionic radius: a) Na, Na+ b) S, Mg c) F, F-, F+ d) K, Rb, Fr e) O, O222) Write the electron configuration AND orbital notation for the following: a) Co b) Si c) Na 23) 24) 25) 26) The following questions relate to the flame test lab: What was unknown A? What was unknown B? How could you tell? Which part of the substance caused the color change, the metal or the nonmetal? How do you know? Put the known substances that you used in order of increasing energy. Describe the relationship between electron affinity, electronegativity, and ionization energy using fluorine and francium as examples. YOU MUST draw electron configurations AND orbital notations to gain full credit.