Unit 3 - Periodic Trends and Spectroscopy Test Review
advertisement

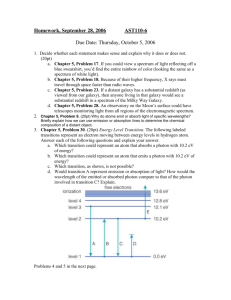
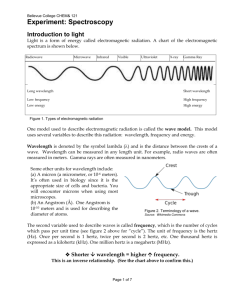
Unit 3 - Periodic Trends and Spectroscopy Test Review Objectives: 1. Apply your understanding of the periodic trends (ionization energy, electronegativity, and atomic radius, effective nuclear charge, and ionic radius) by taking a set of elements in placing them in order by the value of the property. 2. Apply your understanding of ionization energy, electronegativity, and atomic radius and their trends to explain observations or discuss how that property applies to a specific process. 3. Use knowledge of the properties of light, the electromagnetic spectrum, and the structure of the Bohr model of the atom to analyze the relationships between energetic transitions of electrons, wavelength, frequency, and energy. 4. If given c=λν, E=hν, c = 3.00x108 m/s, and h = 6.626x10-34 Js, be able to calculate wavelength, frequency, or energy from any of the other quantities. 5. Use an understanding of the factor label method to correctly convert metric units so that they may be used in subsequent calculations. 6. Use the electromagnetic spectrum on the reference tables to determine which type of light a photon is based on either the wavelength, frequency, or energy. Practice Problems 1. Arrange the following in order of decreasing ionization energy: Sn, S, Ar, Tl, Ra, As, Ba 2. Arrange the following in order of increasing electronegativity: O, Ca, Rb, S, Ge, F, Cs 3. Arrange the following in order of decreasing size: Ar, Cs, As, Sr, P, Ba, Ga, Sc 4. Arrange the following in order of increasing size: Se2-, Sr2+, Zr4+, Kr, Rb+, Br5. Arrange the following in order of increasing effective nuclear charge: Mg, Te, Li, P, Al, At, Be 6. A microwave oven might use an emission at 1.258x1010 Hz to cook your food. Calculate the wavelength and energy of a photon of this light. 7. One of the brightest lines in a copper bright line emission spectrum is 324.8 nm. Calculate the frequency and energy of a photon of this light. What part of the spectrum does this fall in? 8. Bluetooth devices operate in the 2.4GHz range. What is the wavelength and energy of a photon of this light? What part of the spectrum does this fall in?