Final Exam Review
advertisement

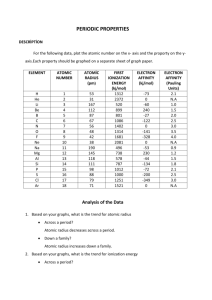
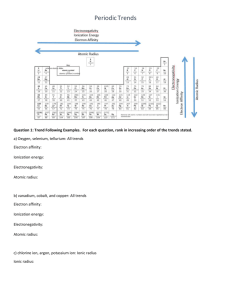
Applicable concepts/equations Etotal = Ephoton* # photons ℎ𝑐 𝐸= l Can a wave do this? ****Things we discussed in this course. Can a Particle do this? yes yes yes no yes no yes no no yes https://www.youtube.com/watch?v=DfPeprQ7oGc Double Split Experiment Does this show that light has wavelike or particle like properties? Why? Wave-like: It creates an interference pattern. What would the results look like if they had particle like properties? Two bright lines behind the splits no interference pattern. Can a wave do this? ****Things we discussed in this course. Can a Particle do this? yes yes yes no yes no Photoelectric Effect Does this show that light has wavelike or particle like properties? Why? What would the results look like if they had only wave-like properties? What is the effect of increasing the intensity of a laser of a frequency less than the threshold frequency? What is the effect of increasing the intensity of a laser of a frequency greater the threshold frequency? http://phet.colorado.edu/en/simulation/photoelectric Schrodinger equation solutions Particle in a box (1d) Particle in a box (2/3D) Didn’t cover Hydrogen Atom Multi electron atoms= many types of approximation, no exact solutions Subject of Current research: We saw result, of appoximations For each of the previous neutral electron configurations, give an excited state. Keep same number of electrons, move at least one up in energy: Many many many correct answers. Effective Nuclear Charge Electronegativity Ionization Energy Electron Affinity Atomic Radius Electron Affinity Ionization Energy Electronegativity Atomic Radius Effective Nuclear Charge Electronegativity Ionization Energy Electron Affinity Atomic Radius Electron Affinity Ionization Energy Electronegativity Atomic Radius Write the electron configurations for C, N, and O. Place in order of increasing ionization energy, increasing electron affinity and increasing electronegativity. For each characteristic, do these follow the trend? Why or why not? Wrong Answer 1: All: C, N, O Wrong Answer 2: Variety of wrong/right answers Yes No Because the trend goes up and to the right. But hydrogen is half filled. Because the trend goes up and to the right. Hint: what is the p block exceptions to electron affinity? Hint: What would be the equivalent of that with the dblock? Which group do these elements belong to? First is always smallest, The “jump” indicates stable configuration I1 Element 1 I2 0.605 1.110 Element 2 0.203 3.215 I3 I4 1.45 5.10 3.89 4.42 Why do we see the sun as different colors? Which lights depicted in this diagram are emitted, which are scattered. What causes the events depicted by the blue arrows in the atmosphere. CN C N 2p E CH3OH can be synthesized by the reaction shown. What volume of H2 gas (in L), at 748 mmHg and 86 oC, is required to synthesize 25.8 g CH3OH? 𝐶𝑂 𝑔 + 2𝐻2 𝑔 → 𝐶𝐻3𝑂𝐻 Grams product moles product conversion moles reactants conversion Volume Reactant Ideal gas law Part 2: If the reaction is known to only be 70.0% efficient (aka has a 70% yield) what volume is required (at the same conditions)?