Rally Quiz
advertisement

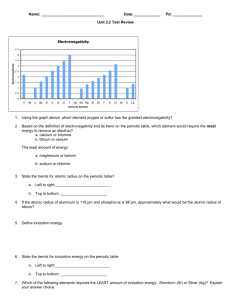
AP Rally Quiz 1 Names: ______________________________________________ The stratospheric ozone (O3) layer helps to protect us from harmful ultraviolet radiation. It does so by absorbing ultraviolet light and falling apart into an O2 molecule and an oxygen atom, a process known as photodissociation. O3(g) O2(g) + O(g) Use the data in Appendix C to calculate the enthalpy change for this reaction. What is the maximum wave-length a photon can have if it is to possess sufficient energy to cause this dissociation? In what portion of the spectrum does this wavelength occur? Microwave ovens use microwave radiation to heat food. The microwaves are absorbed by moisture in the food, which is transferred to other components of the food. As the water becomes hotter, so does the food. Suppose that the microwave radiation has a wavelength of 11.2 cm. How many photons are required to heat 200 mL of coffee from 23°C to 60°C? Use electron configurations to explain the following observations: a. The first ionization energy of phosphorus is greater than that of sulfur. b. The electron affinity of nitrogen is lower (less negative) than those of both carbon and oxygen c. The second ionization energy of oxygen is greater than that of fluorine. d. The third ionization energy of manganese is greater than those of both chromium and iron.