Light and Energy Calculations
advertisement

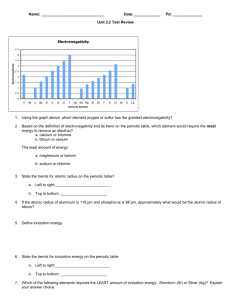
Light and Energy Calculations 1. A certain photon of light has a wavelength of 422 nm. What is the frequency of the light? 14 2. What is the energy of a quantum of light with a frequency of 7.39 x 10 Hz? 3. What is the wavelength of the quantum of light in question 2? 14 4. A certain blue light has a frequency of 6.91 x 10 Hz. What is the wavelength of the light? 5. If it takes 3.36 x 10-19 J of energy to eject an electron from the surface of a certain metal, calculate the longest possible wavelength, in nanometers, of light that can ionize the metal. 6. Ionization energy is the energy required to remove an electron from an atom in the gas phase. The ionization energy of gold is 890.1 kJ/mol. Calculate the minimum wavelength of light that will ionize gold. 7. Spectral data from classroom observations: Use your data, or use the average wavelengths below. Calculate the frequency for each band. What do you think would be the energy released by the electron for each band? a. Mercury orange – 615 nm green – 549 nm violet – 438 nm b. Argon violet – 404 nm indigo – 436 nm blue – 488 nm green – 514 nm orange - 618 nm red – 698 nm c. Krypton violet – 409nm indigo – 437 nm blue - 458 nm aqua – 475 nm green – 522 nm yellow – 557 nm orange – 599 nm red – 642 nm 8. Which color light has the longest wavelength? Which has the highest energy? 9. The energy of the electrons in each of the first 5 energy levels is shown in the table below. Energy level Energy value 1 -13.6 eV 2 -3.4 eV 3 -1.51 eV 4 -.85 eV 5 -.54 eV As you can see, additional energy that it takes to jump from one energy level to the next decreases as you move outward. The reason for this difference is that the photon of light (quantum) that is absorbed as the electron moves outward or given off as the electron drops inward is a different wavelength for each step. This explains why for hydrogen only one color band is seen in the spectral emission, while for potassium, with three different energy levels there would be three bands. As an electron moves from energy level one to energy level two how much energy must be absorbed? From energy level 3 to energy level 4? If the electron drops back to the 1st energy level what color light would be given off? (Planck’s constant in eVs is 4.14 x 10-15) What if an electron dropped from energy level 5 to energy level 4? How much energy would be given off? What wavelength would it be? What color band would be seen? 10. How many of each subatomic particle are in each of the following? a. Neutral chlorine atom b. The Al3+ ion c. U-242 atom d. I – 131 ion with a charge of -1