2010
advertisement
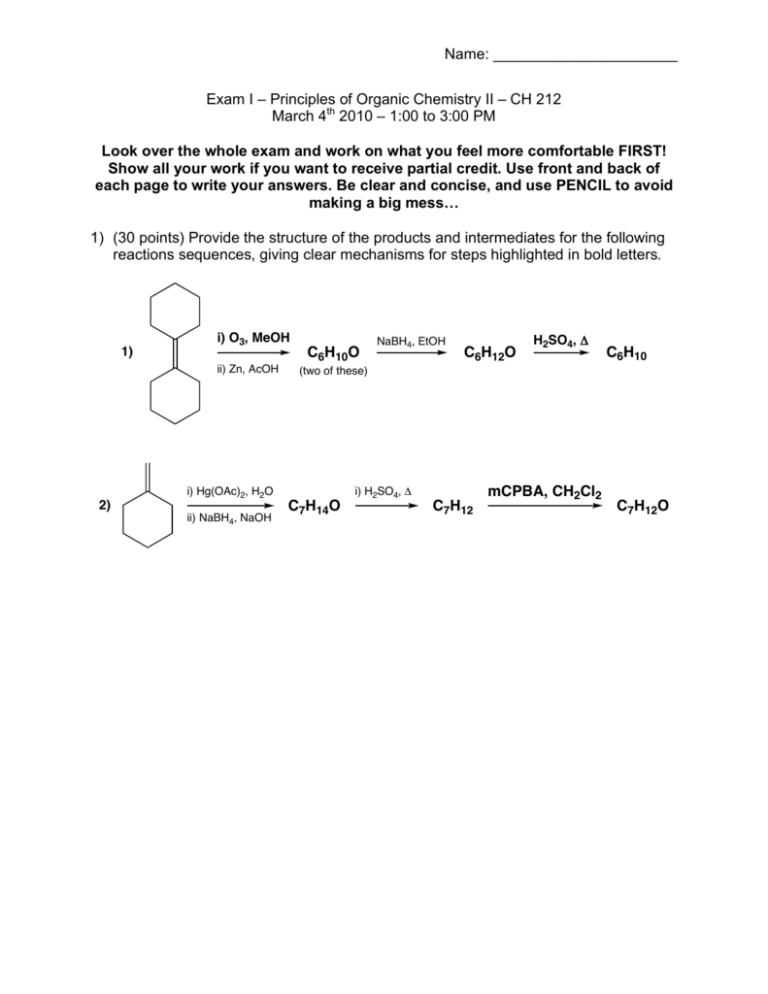
Name: ______________________ Exam I – Principles of Organic Chemistry II – CH 212 March 4th 2010 – 1:00 to 3:00 PM Look over the whole exam and work on what you feel more comfortable FIRST! Show all your work if you want to receive partial credit. Use front and back of each page to write your answers. Be clear and concise, and use PENCIL to avoid making a big mess… 1) (30 points) Provide the structure of the products and intermediates for the following reactions sequences, giving clear mechanisms for steps highlighted in bold letters. 1) i) O3, MeOH ii) Zn, AcOH 2) i) Hg(OAc)2, H2O ii) NaBH4, NaOH C6H10O NaBH4, EtOH C6H12O H2SO4, ! C6H10 (two of these) C7H14O i) H2SO4, ! C7H12 mCPBA, CH2Cl2 C7H12O Name: ______________________ 2) (20 points) Consider the following chlorinated hydrocarbons (A and B): Cl Cl A B a) Write the mechanism for the methanolysis (solvolysis in methanol) of both compounds. b) Which of the two will undergo methanolysis faster? Explain this clearly based on the structure of the intermediates proposed in part (a). Name: ______________________ 3) (20 points) Benzoin can in principle exist in equilibrium between keto and enol forms. OH O OH OH Benzoin Based on the 1H NMR spectrum of benzoin shown below, to which side is the equilibrium displaced? Your answer should be supported by careful interpretation of the spectrum to receive full credit. Name: ______________________ 4) (30 points) For the following three compounds: O 1) O 2) O N OH O O O 3) N O a) Calculate their degree of instauration, and show in the structure what gives rise to this number (i.e., double bonds, rings, etc…). b) Determine how many 1H and 13C NMR signals you will find in their spectra (i.e., identify magnetically equivalent and non-equivalent nuclei). c) For each magnetically equivalent set of protons, specify their multiplicity, assuming that all coupling constants are equal. d) To which of the three compounds shown above do the 1H and 13C NMR spectra provided in the attached pages belong? Name: ______________________ 5) (30 points) A symmetric alkene of formula C12H20 (A) is subjected to the following reactions to obtain another alkene of formula C6H10 (D): C12H20 (A) i) O3, MeOH ii) Zn, AcOH C6H10O (B) NaBH4, EtOH C6H12O (C) H2SO4, ! C6H10 (D) (two of these) 1 H NMR, 13C NMR, and IR data for compounds B, C, and D can be found on the following pages. Using the information provided, give the structures for compounds A through D. You do not need to write all the reaction mechanisms, but have to assign two or more signals in the 1H NMR and/or IR spectra of each compound. You can do that in the spectra if you want, but if you do make sure to turn them in together with the rest of the exam. Hint: I told you not to worry about the mechanisms, but you did some of them earlier in the exam… Name: ______________________ Name: ______________________ 1 1 7 1 2 Problem 4 4 1 Problem 5 Compound B (C6H10O) 1H & 13C NMR Additional peak At 212 ppm… 2 4 4 Problem 5 - Compound B (C6H10O) - IR Problem 5 Compound C (C6H12O) 1H & 13C NMR 5 1 2 2 1 1 Problem 5 - Compound C (C6H12O) - IR Problem 5 - Compound D (C6H10) - 1H & 13C NMR 4 4 2 Problem 5 - Compound D (C6H10) - IR









