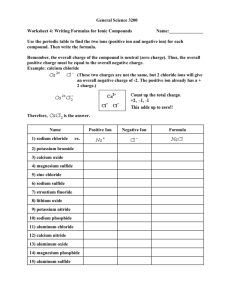
Solution Stoichiometry WS#1

ADVANCED CHEMISTRY – SOLUTION STOICH – WS #1
1.
You have a solution of table salt, sodium chloride, in water. What happens to the salt concentration (increases, decreases, or stays the same) as the solution boils? Draw molecular level pictures to explain your answer.
2.
Which of the following diagrams best represents the hydration of NaCl when dissolved in water? The Cl ion is larger in size than the Na + ion. Explain your choice.
3.
Differentiate between what happens when the following are dissolved in water: a.
polar solute versus nonpolar solute b.
KF versus C
6
H
12
O
6
4.
Match each name below with the following microscopic pictures of that compound in aqueous solution: a.
barium nitrate b.
sodium chloride c.
potassium carbonate d.
magnesium sulfate e.
Which picture best represents HNO
3
(aq)? f.
Why aren’t any of the pictures a good representation of HC
2
H
3
O
2
(aq)?
5.
Using solubility guidelines, predict whether each of the following compounds is soluble or insoluble in water.
What ions, if any, are present upon dissolving the substances in water? a.
PbI
2 b.
MgBr
2 c.
(NH
4
)
2
CO
3 d.
Sr(OH)
2 e.
ZnSO
4
6.
You are presented with a white solid and told that due to careless labeling, it is not clear if the substance is barium chloride, lead chloride, or zinc chloride. When you transfer the solid into a beaker and add water, the solid dissolves to give a clear solution. Next a Na
2
SO
4 (aq)
solution is added and a white precipitate forms. What is the identity of the unknown white solid?
7.
Calculate the molarity of each of these solutions: a.
A 5.623 g sample of NaHCO
3
is dissolved in enough water to make 250.0 mL of solution. b.
A 184.6 mg sample of potassium dichromate is dissolved in enough water to make 500.0 mL of solution. c.
A 0.1025 g sample of copper metal is dissolved in 35 mL of concentrated HNO
3
to form Cu 2+ ions, and then water is added to make a total volume of 200.0 mL (calculate the molarity of Cu 2+ ions).
8.
A person suffering from hyponatremia has a sodium ion concentration in the blood of 0.118 M and a total blood volume of 4.6 L. What mass of sodium chloride would need to be added to the blood to bring the sodium ion concentration up to 0.138 M, assuming no change in blood volume?
9.
Describe, using lab equipment and calculations, how you would prepare 250.0 mL of 0.707 M NaNO
3
solution.
10.
Critical Thinking: Indicate the concentration of each ion present in the solution formed by mixing: a.
42.0 mL of 0.170 M NaOH and 37.6 mL of 0.400 M NaOH b.
44.0 mL of 0.100 M Na
2
SO
4
and 25.0 mL of 0.150 M KCl c.
3.60 grams of KCl in 75.0 mL of 0.250 M CaCl
2
. Assume that the volumes are additive.