File - Herriman Science
advertisement

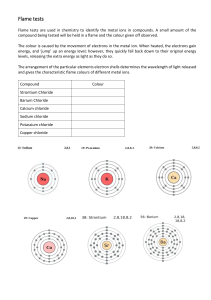
FLAME TEST LAB PROBLEM: How can the identity of an unknown metal be determined? BACKGROUND INFORMATION: When atoms are placed into a flame, electrons absorb energy. This extra energy causes the electrons to jump into an excited energy state, also known as a quantum jump. Because energy must always be conserved, electrons re-emit this energy as a photon when they return to their ground state. The amount of energy in the photon (particle of light) determines its color. The order of energy follows the visible light spectrum with red being the lowest and violet being the highest (red, orange, yellow, green, blue, indigo, and violet) The arrangements of electrons (electron configuration) determine the sizes of the quantum jumps. Remember, quantum jumps are measured by viewing the spectrum of color emitted by the photon when the electrons return to their ground state. When viewed with the naked eye, the spectrum will appear all one color. However, we can separate out the light into its spectrum using a spectroscope. This spectrum helps us determine which element is present. In this lab, we will record the flame color test for various metals by soaking a wood splint in water, adding the salts to the splints and putting the salt and splint into a flame. Finally we will use the data to try to identify a metal in an unknown salt solution. HYPOTHESIS: _______________________________________________________________________________ PRE-LAB 1. Write the electron configurations for the following elements Sodium _________________________________________________________ Calcium _________________________________________________________ Lithium _________________________________________________________ Strontium _______________________________________________________ Potassium _______________________________________________________ Copper __________________________________________________________ Chlorine _________________________________________________________ Sulfur ___________________________________________________________ Nitrogen ________________________________________________________ Oxygen _______________________________________________________ 2. Explain Conservation of Energy. MATERIALS: Metal salt solutions (CaCl2, CuCl2, LiCl, KCl, SrCl2-6H20, NaCl, Unknown) Bunsen Burner 7 wooden sticks (soaked in water) 100 mL water 7 weighing boats PROCEDURE: 1. Fill 100 mL beaker half full with water. Place the 7 wooden sticks in the water to continue to soak. 2. Fill a second 100 mL beaker with water, label this “rinse water” 3. Label 7 weighing boats Ca, Cu, Li, Na, K, Sr and unknown. Place one scoopful (about 0.5 g) of each solid metal chloride into corresponding weighing boats 4. Light the Bunsen burner, adjust until flame is blue. 5. Dip the soaked end of wooden stick into one of the metals. Place in to flame and observe color. Be careful not to drop any of the salt into the barrel of the Bunsen burner, as it will give you a large degree of error. 6. Extinguish flame by placing wooden stick in “rinse water” beaker. 7. Record observations in data table 8. Repeat with the other metals 9. Follow steps 5-7 to test the unknown and use the data from the prior metal solutions to try to hypothesize what the unknown metal is 10. Clean up station. Throw left over salts and sticks in the trash. Flush rinse water down the sink. Wash and dry equipment. Wipe down station. DATA: Sample CaCl2 Calcium chloride CuCl2 Copper(II) chloride LiCl Lithium chloride KCl Potassium chloride SrCl2 Strontium chloride NaCl Sodium chloride Unknown Table 1.1 Metal Flame color *Wavelength values here are given for the mid-range of the color indicated. ANALYSIS Table 1.1 Sample Metal/ cation Flame color Wavelength (nm) 𝒉𝒄 𝝀 1m=10-9nm 𝑬= Wavelength (m) Energy (J) CaCl2 Calcium chloride CuCl2 Copper(II) chloride LiCl Lithium chloride KCl Potassium chloride SrCl2 Strontium chloride NaCl Sodium chloride Unknown 1. Based off the color you observed, use Table 1.1 to record your wavelength in nanometers (nm). 2. Convert your wavelength from nanometers (nm) to meters (m) and record in the data table above. Show your work for at least one conversion. 3. Using the wavelength in meters, calculate the Energy in Joules (J) using the following equation and constants (show your work). 𝒉𝒄 𝑬= h= 6.63x10-34Js c=3.00x108 m/s 𝝀 4. Explain what is happening to the electrons at the atomic level? Make sure to include what excites the electrons and how the different colors of light are produced. 5. What was your unknown? Explain how you reached this conclusion. Reflection: Write a paragraph discussing the lab: what worked, what didn’t work, what you learned and how this lab relates to the material discussed in class. Category Procedure Data/ observations Analysis Reflection Lab Rubric Description Did you answer your pre-lab questions? Did you follow procedures correctly? Did you adhere to all safety protocol? Did you wear your apron and goggles? Did you clean up and dispose of chemicals properly? Did you record your data accurately? Do you have a table to display your data? Do you understand what you observed? Can you draw a conclusion? Are your analysis values visible? Do you show proper significant digits and unit of measurements? Did you quantify your calculations correctly? Did you use complete sentences? Are there at least 5 sentences? Did you mention your possible reasons for error? Did you mention what you did well? Could improve on? Did you say what you learned? Total (30 pts) 20% (6 pts) 15% (4 pts) 45% (14 pts) 20% (6 pts) Your score