Chem 362 February Midterm 2012 Smith
advertisement

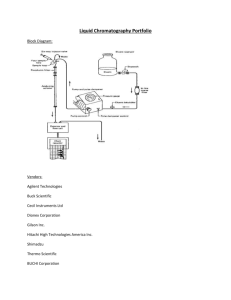
1 Chem 362 February Midterm 2012 Smith-Palmer Name: The overall principle behind chromatography is differential hold up. [2] 1. Name 5 different ways to achieve this. Adsorption Partition Affinity Ion exchange Molecular exclusion [1] 2. Which of these ways is important in reverse phase HPLC and in gas liquid chromatography? [2] 3. Draw a labeled block diagram for an HPLC. loop injector solvent reservoirs [2] pumps column UV-vis absorption detector 4. Draw a labeled block diagram for a GLC. oven gas tank & regulator column injector & vaporizer FID 2 [4] 5. In gas chromatography, the parameter that is changed during a gradient run is temperature………………………… Should we increase or decrease it during a run? Increase In liquid chromatography with a reverse phase column, the parameter that is changed during a gradient run is Polarity Should we increase or decrease it during a run? Decrease [4] Is it easier/more convenient to run a gradient in gas chromatography or liquid chromatography. Why? HPLC columns take longer to equilibrate When should you use a gradient? When you have a mixture of very different compounds – and there are some later ones that are all well separated If its a long run for HPLC so equilibration time doesn’t matter [3] 6. Why do bands get broader in a chromatogram as retention time increases? More diffusion because of the time the analyte spends on the column Why is this worse in gas chromatography than in liquid chromatography? Sample is a gas, high temperatures 3 [5] 7. Calculate N and H using the second peak of the following chromatogram. The column used was 12 m in length. First peak: retention time = 60 s, half width = 6.0 s Second peak: retention time = 100 s, half width = 10 s N = 5.55 × 1002 = 5.55 × 102 (5.611 × 102) 102 H = 1200 cm = 2.2 cm (2.1 cm) 555 What is the resolution between the two peaks? Rs = 0.589 × 40 s = 2.945 8s = 2.9 [1] Suggest what mobile phase might have been used. N2 gas [2] What instrument settings could be changed to decrease the resolution? Increase flow rate, increase temperature 4 [4] 8. With respect to an ion selective electrode, what does the selectivity coefficient tell us? Tells us how much other ions interfere Is it better to have a large or a small selectivity coefficient? Explain Small – as in way less than 1 This means that the response to another ion is way less [4] [1] Draw a labeled diagram to show how an ISE can be modified and used to detect carbon dioxide. Which is more sensitive – an ISE for calcium ions or an ISE for lithium ions? Lithium ions 5 [5] 9. Calculate the cathodic potential when a dilute sodium sulfate solution , pH 8.0, is electrolyzed. The cathode is a smooth platinum electrode with a surface area of 1 x 10-3 m2. The current is 0.100 A and the product is hydrogen gas at 1.00 bar. TABLE: Overpotential (V) for hydrogen evolution at various current densities at 25oC Electrode Current density (A/m2) 10 100 1000 10000 Smooth Pt 0.024 0.068 0.288 0.676 2H+ + 2e– → H2 Ec = 0 = 0.059 V log 1 2 (10–8)2 = 0 – 0.059 V × 8 = –0.47 V Overall Ec = –0.47(2) – 0.068 = – 0.54 V [4] 10. Name four different parameters that are important in determining the size of an overpotential. Current density Gas production Number of e– transferred Nature of metal Temperature 6 [2] 11. Calculate the ionic strength of a solution containing 0.3 M aluminum chloride. = ½ (0.3 × 9 + 0.9) = ½ × 3.6 = 1.8 M =2M [1] Will the activities of these solutions be larger or smaller than their concentrations? Smaller [3] 12. In polarography, what produces the charging current? (What causes it?) 7 [2] What does it mean if we say an electrode is partially polarized? Current is not linearly dependant on V [2] What does it mean if we say an electrode is completely polarized? i doesn’t depend on V [2] Why is complete polarization of the mercury electrode important in polarography? id is proportional to [analyte] 8 [3] [2] Draw the voltage versus time plot for the voltage that is applied in square wave polarogaphy. What is the advantage of square wave polarography as compared to differential pulse polarography? Fast, better sensitivity [2] Draw the shape you would expect to see for the cyclic voltammogram of a reversible reaction. Label the axes. I like ducks. i v