CHEMISTRY 110
advertisement

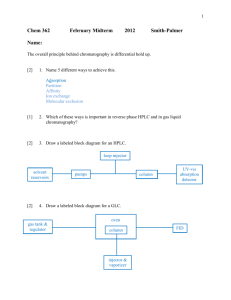
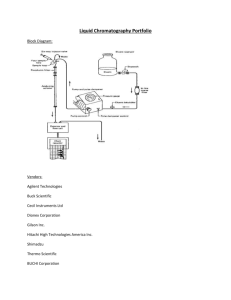
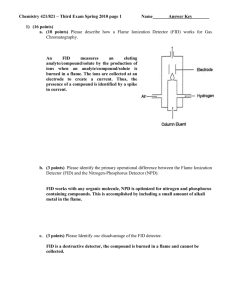
Chemistry 421/821 – Third Exam Spring 2009 page 1 Name Answer Key 1) (6 points) Briefly describe how the following chromatography methods address the general elution problem: a. Liquid Chromatography Gradient elution – change composition of mobile phase with time. b. Gas Chromatography Temperature gradient – change temperature with time. c. Supercritical Fluid Chromatography Pressure gradient – change temperature with time. 2) (15 points) Please describe how a Thermal Conductivity Detector (TCD) works for Gas Chromatography. Please Identify one advantage and one disadvantage of the TCD detector. Measure changes in thermal conductivity as analyte elutes from column., different ability of solvent and analyte to conduct heat away from hot wire. TCD uses a Wheatstone bridge, a circuit of matched resistors under a constant current and zero potential. Resistors are temperature dependent. Column eluent passes over one resistor and a reference stream passes over a second resistor. Any change in the thermal conductivity of the streams passing over the two resistors results in a change in temperature. Since resistance changes as a function of temperature, an observed voltage change occurs as a compound elutes from the column. Advantages – universal and non-destructive Disadvantages – high limit of detection, sensitive to flow rate changes and impurities in mobile phase Chemistry 421/821 – Third Exam Spring 2009 page 2 Name Answer Key 3) (15 points) Please describe the fundamental differences between Ion-Exchange Chromatography and Size-Exclusion Chromatography. Ion-Exchange Chromatography separates analytes by charge where Size-Exclusion Chromatography separates based on size. No stationary phase, strong or weak mobile phases for Size-Exclusion. Ion-Exchange Chromatography – separates solutes by their adsorption onto a support containing fixed charges on its surface. Thus, retained analytes are ions with opposite charges relative to the stationary phase. The strong mobile phase contains a high concentration of competing ions to displace the sample ion from the stationary phase Size-Exclusion Chromatography – separates molecules according to differences in their size. Lower MW compounds elute slower because they travel a longer path through pores in the support material. Larger MW compounds cannot fit into the pores and remain in the flowing mobile phase. No true stationary phase, No strong or weak mobile phase, separation based on shape. 4) (5 points) Please identify one typical approach by which a ligand is eluted from a column after being retained in affinity chromatography. Change pH or buffer to decrease affinity or disrupt complex Add competing compound to displace retained ligand, similar to Ion-exchange chromatography (above). Chemistry 421/821 – Third Exam Spring 2009 page 3 Name Answer Key 5) (15 points) An organic liquid-membrane electrode was developed to selectively measure As3+ in water: (PP)3As 3PP- (organic) + As3+ (organic) aqueous a. (5 points) How will the potential of this electrode vary as a function of [As3+] (i.e., write out a Nernst equation)? Similar to calcium dialkyl phosphate liquid membrane and pH electrode, [PP-] included in c. Divided by three because cation is trivalent. 𝑬𝒊𝒏𝒅 = 𝒄 − 𝟎. 𝟎𝟓𝟗𝟐 𝒑𝑨𝒔 𝟑 b. (5 points) Why would a potential develop across this liquid-membrane electrode if it is placed in contact with a solution containing an unknown concentration of As3+ Concentration difference of As3+ on both sides of membrane charge separation potential c. (5 points) A cell potential of 0.638 V was measured using the above indicator electrode using SCE (0.244 V) as a reference electrode. What would the cell potential measure if a Ag.AgCl (0.199 V) electrode was used instead of SCE as a reference electrode? Ecell = Eind - Eref Thus Ecell would increase by the difference in the two reference potentials 0.244-0.199 = 0.045 Ecell = 0.683 V Chemistry 421/821 – Third Exam Spring 2009 page 4 Name Answer Key 6) (19 points) Given the following electrochemical cell: Ni(s) | NiSO4 (0.0025M || KIO3 (0.10 M) | Cu(IO3)2 (s) | Cu(s) a. (15 points) If the cell potential is 0.512 V, find Ksp for Cu(IO3)2 (ignore activity coefficients). Cu2+ + 2e- Cu(s) Eo = 0.337 V - Ni2+ + 2e- Ni(s) Eo = -0.250 V Cu2+ + Ni(s) Cu(s) + Ni2+ Eo = 0.587 V 𝑬𝒄𝒆𝒍𝒍 = 𝑬𝒐 − [𝑵𝒊𝟐+ ] [𝟎. 𝟎𝟎𝟐𝟓] 𝟎. 𝟎𝟓𝟗𝟐 𝟎. 𝟎𝟓𝟗𝟐 𝒍𝒐𝒈 = 𝟎. 𝟓𝟏𝟐 = 𝟎. 𝟓𝟖𝟕 − 𝒍𝒐𝒈 → [𝑪𝒖𝟐+ ] = 𝟕. 𝟑𝟏𝒙𝟏𝟎−𝟔 𝑴 [𝑪𝒖𝟐+ ] [𝑪𝒖𝟐+ ] 𝟐 𝟐 Ksp = [Cu2+][IO3-]2 = (7.31x10-6 )(0.10)2 = 7.31x10-8 b. (4 points) Identify one potential source of error in the above calculation (Please only refer to errors associated with measuring the cell potential). Liquid junction potentials, non-Faradic current ohmic potential or IR drop, electrode polarization effects Chemistry 421/821 – Third Exam Spring 2009 page 5 Name Answer Key 7) (15 points) A 0.0809 g sample of an impure organic acid (FW = 247.14) was dissolved in an alcohol-water mixture and titrated with coulometrically generated hydroxide ions. With a current of 0.0441 A, 266 s was required to reach a phenolphthalein end point. a. (10 points) Calculate the percent purity of the acid. Q = it = (0.0441 A)(266 s) = 11.7306 (11.7306 C)(1 mole e-/96,487)(1 mol HA/1 mol e-)(247.14 g HA/mol) = 0.0300g Percent purity = (0.0300g/0.0809 g)*100 = 37.14% b. (5 points) What is the most likelihood source of error in the above calculation? Departure from 100% current accuracy, all the current wasn’t used to titrate acid. 8) (10 points) A constant potential of -1.0 V was applied to mixture containing Cu+2 and Cd+2, causing both cadmium and copper ions to be reduced and deposited as metals. The voltage was then slowly reduced given the following voltammogram: a. (5 points) Which metal is oxidized first? Why? Cd, lower oxidizing potential b. (5 points) Which ion had a higher concentration in the original mixture? Why? Cu, higher limiting current, limiting current proportional to concentration (i = kc)