E2 Rev & Key
advertisement

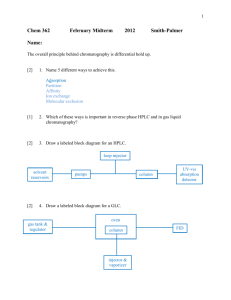
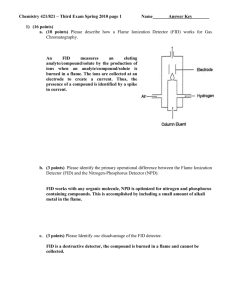
Chem 251 Exam 2 Review 1. What is the main purpose of chromatography? 2. What is the difference between the mobile phase and the stationary phase? 3. In liquid chromatography, which is definitely liquid, the mobile or stationary phase? 4. Describe the difference between normal phase, reverse phase, ion exchange and gel filtration chromatography. 5. Which of the above is the most common in HPLC ? 6. The various theories and equations of chromatography given were all based on a reaction like this: AM AS Where A is the analyte and M and S are the mobile and stationary phases. Explain this reaction and how it relates to chromatography. 7. Given the equations, be able to calculate k, , R, N and HETP from a chromatogram. 8. What is R a measure of? What are some factors that determine R? What’s a “good” value for R? 9. What is a theoretical plate? What are some factors that determine N? 10. Explain the general setup of a GC experiment. 11. What are some typical carrier gases? 12. What is the difference between packed and capillary GC columns? 13. What are WCOT, SCOT, and PLOT? 14. Describe how FID, ECD, and TCD work. 15. Why are high pressures necessary in HPLC? 16. What is a bonded stationary phase? Which is most common in HPLC? 17. Describe Adsorption Chromatography. 18. A weak acid, HA, has a pKa of 5.6. What form predominates at pH 7? How may changing the pH effect t R in HPLC? 19. What is gradient elution and why would you need to use it? What types of gradients may be used? 20. What is the most common detector in HPLC? 21. How can HPLC or GC be quantitative? 22. Describe how to use an Internal Standard in chromatography. 23. In reverse phase chromatography, the ________ (more polar/ less polar) analytes have higher retention times. 24. In the voltaic cell represented as: Cd │ Cd2+ ║ (Pt) Fe3+ │ Fe2+ the electron flow will be from: a. Pt to Cd2+ b. Pt to Cd c. Fe2+ to Cd2+ d. Cd2+ to Fe2+ e. Cd to Fe3+ 2+ 2+ 25. For the voltaic cell represented as: Zn (s) │ Zn (1.0 M) ║ Cu │ (1.0 M) Cu (s) Which of the following statements is false ? a. The mass of the zinc electrode decreases. b. The copper electrode is the anode. c. Electrons flow through the external circuit from the zinc electrode to the copper electrode. d. Reduction occurs at the copper electrode. e. The cell is under standard conditions. 26. In a voltaic cell, Zn is oxidized to its ion while chlorine gas is reduced to chloride. The emf is 0.853 V. Calculate the maximum electrical work generated when 2.0 g Zn is consumed. 27. What is the cell reaction for the voltaic cell: Zn (s) │ Zn 2+ ║ Cu+ │ Cu (s) ? 28. What is the emf at 25ºC for the following cell: Cr │Cr 3+ (1.0 x 10 -4 M) ║ Ag + (1.0 x 10 -2 M)│ Ag 29. Calculate Eº for the cell reaction 2Cr + 3Sn4+ -----> 3Sn2+ + 2Cr3+ 30. Consider the following electrode potentials: Mg2+ + 2e -----> Mg Eº = - 2.37 V V2+ + 2e -----> V Eº = - 1.18 V 2+ + Cu + e -----> Cu Eº = + 0.15 V Which one of the reactions below will proceed spontaneously from left to right? a. Mg2+ + V -----> V2+ + Mg b. Mg2+ + 2Cu+ -----> 2Cu2+ + Mg 2+ + 2+ c. V + 2Cu -----> V + 2Cu d. V + 2Cu+ -----> V2+ + 2Cu2+ e. none of these 2+ 31. Which of the following species will spontaneously oxidize Fe to Fe a. Cu 2+ b. Al 3+ c. Mg 2+ d. Zn 2+ e. none of these 32. According to the following cell notation, which species is undergoing oxidation? Sn │Sn 2+(Pt) ║ MnO2 │ Mn 2+ 33. Given the following reaction: MnO4 - + C2O42- -----> Mn2+ + CO2 a) What is the oxidizing agent? b) Give the balanced reaction for acidic conditions. c) Give the balanced reaction for basic conditions. 34. When are you required to use the Nernst equation to find cell voltage? 35. What is potentiometry? 36. What is the difference between a reference and indicator electrode? 37. Describe the Ag-AgCl and Calomel reference electrodes. 38. What are ion selective electrodes and how do they work? 39. Describe how a glass pH electrode works. Answers to Selected Problems 3. mobile 5. reverse 18a. A- (conjugate base) 23. less 24. e 25. b 26. 5.0 kJ 28. 1.50 V (note: there was a typo on this problem originally, see previous page for corrections.) 29. 0.879 V 30. e (d is not the answer since both 1/2 rxns are oxidations which is impossible.) 31. a 32. Sn 33a. MnO433b. 16 H+ + 2 MnO4- + 5 C2O42- --> 2 Mn2+ + 10 CO2 + 8 H2O 33c. 8 H20 + 2 MnO4- + 5 C2O42- --> 2 Mn2+ + 10 CO2 + 16 OH-