13.1 Water and Phase Changes
advertisement
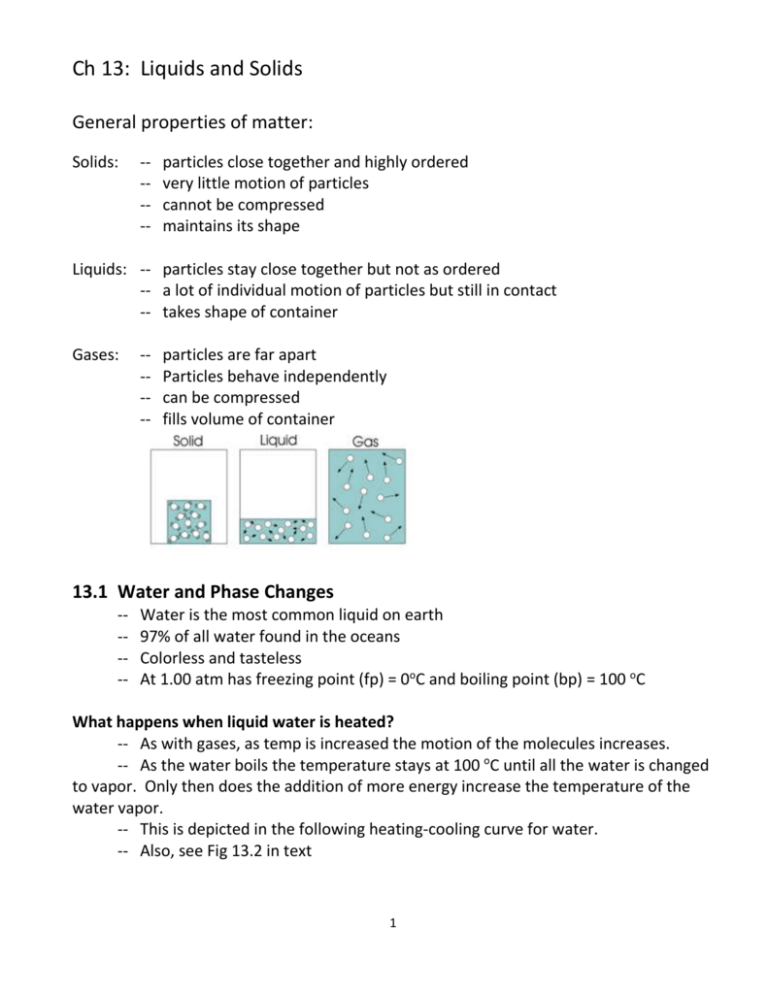
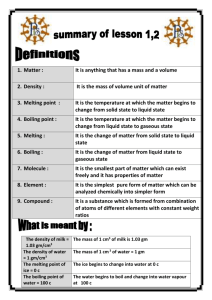
Ch 13: Liquids and Solids General properties of matter: Solids: ----- particles close together and highly ordered very little motion of particles cannot be compressed maintains its shape Liquids: -- particles stay close together but not as ordered -- a lot of individual motion of particles but still in contact -- takes shape of container Gases: ----- particles are far apart Particles behave independently can be compressed fills volume of container 13.1 Water and Phase Changes ----- Water is the most common liquid on earth 97% of all water found in the oceans Colorless and tasteless At 1.00 atm has freezing point (fp) = 0oC and boiling point (bp) = 100 oC What happens when liquid water is heated? -- As with gases, as temp is increased the motion of the molecules increases. -- As the water boils the temperature stays at 100 oC until all the water is changed to vapor. Only then does the addition of more energy increase the temperature of the water vapor. -- This is depicted in the following heating-cooling curve for water. -- Also, see Fig 13.2 in text 1 Temperature oC Energy added Phase changes occur at BC (solid to liquid) and DE (liquid to gas). As mentioned, water temperature does not change during a phase change. -- As ice melts the temperature of the ice-liquid water mixture stays at 0 oC until all the ice has melted (see plateau at 0 oC). -- As water boils the temperature of the liquid water-water vapor mixture stays at 100 oC until all the water turns to vapor (see plateau at 100 oC). Generally, substances are denser as solids than in their liquid forms. This makes sense because as liquids their particles (atoms or molecules) have more energy and are moving around more. The space between particles is greater than when in solid form. Water is an exception. As ice its molecules are locked in position and held slightly further apart than in the liquid phase when there is more motion of the water molecules yet they are closer together. The density of liquid water is 1.00 g/1.00 mL = 1.00 g/ml The density of water ice is 1.00 g/1.09 mL = 0.917 g/mL 2 13.2 Energy Requirements for the Change of State Remember, changes of state (solid to liquid, liquid to gas) are physical, not chemical, changes. No chemical bonds are broken. All physical states for a molecular substance consist of the same molecules. Intramolecular (within the molecule) forces are the bonding forces that hold the atoms of molecules together. These bonding forces can be covalent or ionic (see Ch 11). Intermolecular (between molecules) forces occur between molecules that cause them to aggregate to form a solid or a liquid. (See fig 13.3, 13.4) Intermolecular forces Intramolecular forces (bonds) It takes energy to melt ice and vaporize liquid water because intermolecular forces must be overcome. Once water has been vaporized there is little or no intermolecular force between water molecules. This applies to phase changes for all substances, not just water. The energy required to melt one mole of a substance is called the molar heat of fusion. For ice, the molar heat of fusion is 6.02 kJ/mole. 3 The energy required to vaporize one mole of a liquid is called the molar heat of vaporization. For water, the molar heat of vaporization is 40.6 kJ/mole. Notice, in Fig 13.2 in your text or in the heating/cooling for water above, that the plateau for vaporization is much longer than for melting. In liquid water, the molecules are still fairly close together, so most of the intermolecular forces are still present. However, to separate the molecules to form a gas, virtually all of the intermolecular forces must be overcome, and this requires much more energy. Example 1: How much energy is needed to melt 2.00 moles of ice at 0 oC? Example 2: How much energy is needed to melt 75.0 grams of ice at 0 oC? Example 3: How much energy (in kJ) is needed to heat 25.0 g of liquid water at 50.0 oC to water vapor at 125 oC? 4 13.3 Intermolecular Forces How do intermolecular forces arise? There are several mechanisms 1. Dipole-dipole attraction. -- When molecules with dipole moments (i.e., centers of positive and negative charge) are put together, they orient themselves so that the positive end of one molecule is lined up with the negative end of another. See Fig 13.5 in text. -- Dipoles find the best compromise between attraction and repulsion. -- A dashed line is used to indicate the dipole-dipole attraction -- Examples: -- Dipole-dipole forces are typically only about 1% as strong as covalent or ionic bonds, and become weaker as the distance between dipoles increases. In the gas phase these forces are, therefore, relatively unimportant. 5 2. Hydrogen bonding. -- These are particularly strong dipole-dipole forces between molecules in which H is bonded to a very electronegative atom, such as O, N or F. (See above for HCl and H2O.) -- Hydrogen bonding has important effects on physical properties, such as boiling points and vapor pressure. Bond O-H N-H S-H ∆EN 1.4 0.9 0.4 Molecule water ammonia H2 S BP 100 oC -33.3 oC -60 oC 3. London Dispersion forces -- Even molecules without dipole moments, and even individual atoms, must exert forces on each other. This is known because even substances such as noble gases can exist in the liquid and solid phases at very low temperatures (see Table 13.2). Therefore, there must be forces that hold them together. These forces are known as London dispersion forces. -- These forces occur because atoms can develop a temporary dipolar arrangement of charge as electrons move around the nucleus. -- This instantaneous dipole can induce a similar dipole in a neighboring atom or nonpolar molecule -- This interatomic or intermolecular attraction is weak and short-lived, but can serve to hold these atoms or molecules closer together when temperatures are every low. 6