Phases and Equilibrium Website 3
advertisement

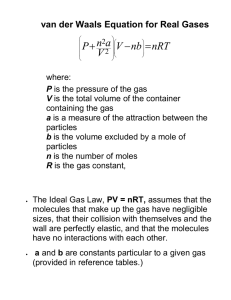
Phases and Equilibrium The States of Matter, Molecular Stickiness, and Thermodynamics The phases of matter represent 'classes' of the type of molecular motion found at different temperatures. When the temperature is low, the motion of molecules is dominated by the fact that they stick together, and the result is a phase of matter that is rigid and dense. When the termperature is high, the motion of the molecules is dominated by their translational energy, so intermolecular forces can almost be ignored. At intermediate temperatures, molecules translate but still stick together. Solids (tightly-bound molecules) At low temperatures the nuclei of the atoms of a solid vibrate about an equilibrium position but are trapped in their lattice positions, unable to flow or diffuse. The intermolecular forces are stronger than the average thermal energy of the system. Long range radial and angular order (structure) are usually present in single crystal solids. Even amorphous solids have relatively good spatial ordering, especially over small distances, (10-100 molecules) Liquids As the binding energy to the lattice site is overcome by thermal energy, the molecules in the solid may slip past each other but maintain close contact. The overall substance is fluid, but not very compressible. Some long range radial ordering persists, but usually only over the size of a few molecular diameters Gases (free motion) Gases are described by the Kinetic Theory of Gases. In this limit, gas molecules have negligible size, have no appeciable intermolecular forces, and are in continous, random motion. Gases have mean free paths that are larger than molecular diameters, i.e. they are usually isolated but occasionally have collisions The state of a gas is universally, if approximately, described by the Ideal Gas Equation of State.