Polar, Nonpolar & Ionic Bonds
advertisement

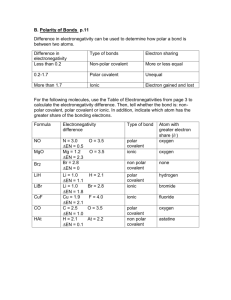
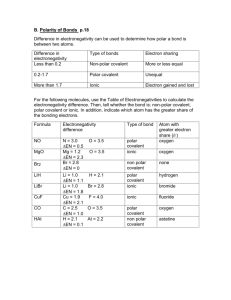
NPHS Chem H Name Date Per. Problem Set: Polar, Nonpolar, and Ionic Bonds 1. Distinguish between polar and nonpolar bonds. 2. Why is an HCl molecule polar while a Cl2 molecule is nonpolar? 3. Why do we show only partial charges, and not full charges, on atoms of a polar molecule? 4a) Which of the following pairs of elements are most likely to form ionic bonds? (show why using electronegativity values!) Te and H C and F Ba and F N and F K and O 4b) Of the remaining 3 pairs of elements, which one forms the least polar, and which the most polar, covalent bond? (show why using electronegativity values) 5) Classify the bonding between the following pairs of atoms as ionic, covalent very polar, covalent moderately polar, or covalent nonpolar (show with electronegativity values and differences WHY you classify each one): a) Si and O f) Sr and F b) N and O g) As and As c) Li and O h) N and F d) Br and I i) Ca and H e) O and O j) H and O 6a) Write Lewis dot formulas for atoms of strontium, chlorine, and silicon. 6b) Use the appropriate pairs of these atoms to construct the Lewis structures of an ionic compound (SrCl2) and a covalent molecule (SiCl4). a) SrCl2 b)SiCl4 7) Draw Lewis structures for the following covalent compounds, and label the positive and negative ends of regions of the molecule (if applicable): a) H2O d) CO32- b) O2 e) HCO3- (hint: C is bonded to all O, O bonded to H) c) NCl3 f) ClO3-