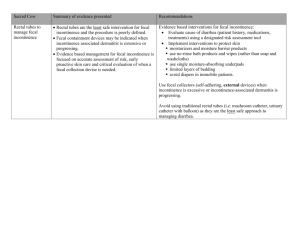
A study of the efficacy of porcine dermal collagen (Permacol
advertisement

A study of the efficacy of porcine dermal collagen (Permacol) injection for passive fecal incontinence in the Colorectal Unit (CRU) at the Wits Donald Gordon Medical Centre (WDGMC). N Harran -Colorectal Fellow (WDGMC), J Herold, B Bebington, D Lutrin. Introduction: Between 1 and10% of adults are affected with fecal incontinence (FI). FI can result from different mechanisms. We are reviewing patients who have sphincter intact FI. There are currently limited treatment options available for this pathology. Conservative methods which include dietary modifications, constipating agents and biofeedback are used with limited benefit. Permacol injection is the only alternative surgical option available to these patients, other than a permanent stoma,. The international literature has shown a good outcome in patients who are correctly selected. There is currently no South African data available on the long term efficacy of this procedure. Research Question: The aim of this study is determine objectively if our patients with sphincter intact FI or passive FI have an improvement in their symptoms post Permacol injection and if so, for how long does the effect last. Methods and Materials: In phase I, patients who had the procedure between the 1st of January 2012 and the 31st of December 2013 were assessed retrospectively. They were asked to complete a selfadministered Wexner and FI QoL score based on their symptoms pre operatively and a second self-administered Wexner and FI QoL score post operatively. These results were then analysed to assess the outcome of the procedure and determine the degree of benefit, if any. In phase II, patients will be enrolled prospectively. Recruitment commenced on the 1st of April 2014 and will be close on the 31st of December 2015. Patients will be asked to complete self-administered Wexner and FI QoL scores pre operatively and at intervals of 1, 3, 6, 12, and 18 months, to assess degree and duration of response. Both phase I and phase II have been approved by the Wits HREC. Conclusion: The results from phase I will be available for presentation. Phase II data and results will only be available in 2016. As there have been no local studies to assess the efficacy of Permacol it will be interesting to see if our findings are of a similar nature to those described in the few international trials on this procedure.