LIST OF APPROVED DRUG FROM 01-01
advertisement
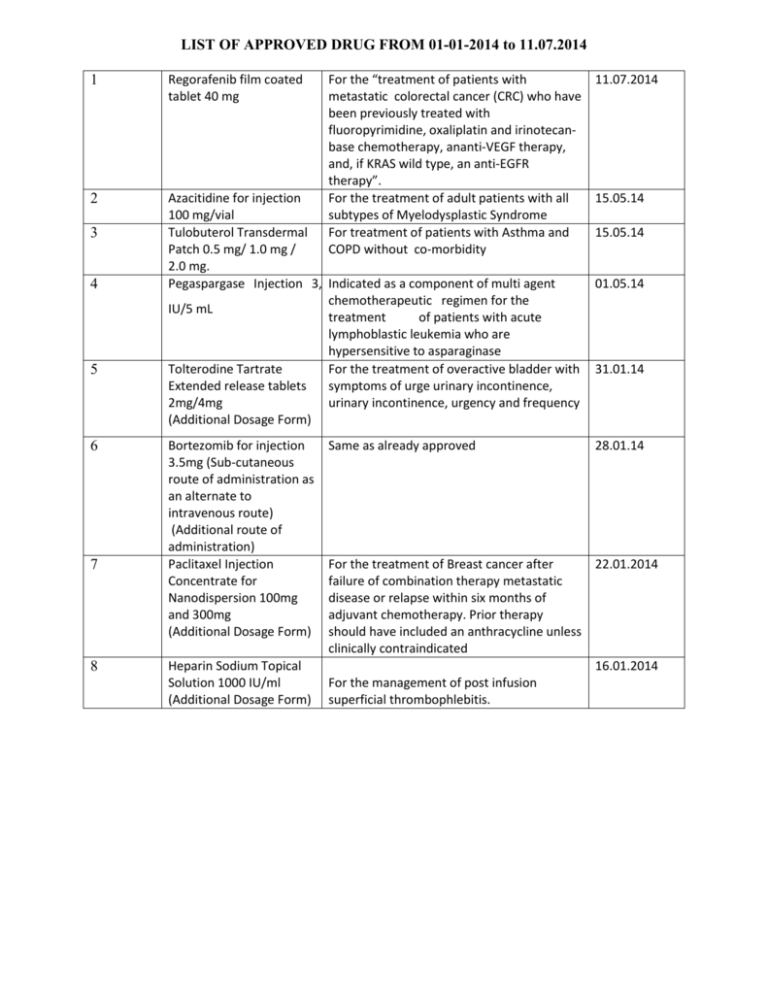
LIST OF APPROVED DRUG FROM 01-01-2014 to 11.07.2014 1 Regorafenib film coated tablet 40 mg 2 Azacitidine for injection 100 mg/vial Tulobuterol Transdermal Patch 0.5 mg/ 1.0 mg / 2.0 mg. Pegaspargase Injection 3,750 Indicated as a component of multi agent chemotherapeutic regimen for the IU/5 mL treatment of patients with acute lymphoblastic leukemia who are hypersensitive to asparaginase Tolterodine Tartrate For the treatment of overactive bladder with Extended release tablets symptoms of urge urinary incontinence, 2mg/4mg urinary incontinence, urgency and frequency (Additional Dosage Form) 3 4 5 6 7 8 Bortezomib for injection 3.5mg (Sub-cutaneous route of administration as an alternate to intravenous route) (Additional route of administration) Paclitaxel Injection Concentrate for Nanodispersion 100mg and 300mg (Additional Dosage Form) Heparin Sodium Topical Solution 1000 IU/ml (Additional Dosage Form) For the “treatment of patients with 11.07.2014 metastatic colorectal cancer (CRC) who have been previously treated with fluoropyrimidine, oxaliplatin and irinotecanbase chemotherapy, ananti-VEGF therapy, and, if KRAS wild type, an anti-EGFR therapy”. For the treatment of adult patients with all 15.05.14 subtypes of Myelodysplastic Syndrome For treatment of patients with Asthma and 15.05.14 COPD without co-morbidity Same as already approved 01.05.14 31.01.14 28.01.14 For the treatment of Breast cancer after 22.01.2014 failure of combination therapy metastatic disease or relapse within six months of adjuvant chemotherapy. Prior therapy should have included an anthracycline unless clinically contraindicated 16.01.2014 For the management of post infusion superficial thrombophlebitis.











