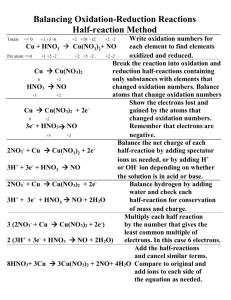
SPECTRA OF SCIENCE Chapter 6 Learning Targets
advertisement

SPECTRA OF SCIENCE Chapter 6 Learning Targets Directions: Use the rating scale below to determine how well you know and can perform each of the learning targets below. Rating Scale: 0 What’s that? 1 I know a little bit 2 I know a lot 3 I could teach this! # Learning Targets: 1 I can identify the reactants and products of a chemical equation. 2 I can identify signs that a chemical reaction has occurred. 3 I am able to explain what happens to bonds and atoms during a chemical reaction. 4 Given a chemical equation, I can identify which of the five types of reactions is occurring. 5 I can identify which elements are oxidized and which are reduced in redox reactions. 6 I am able to differentiate between exothermic and endothermic reactions. 7 I am able to correctly balance chemical equations. 8 I am able to list the factors that affect the rate of chemical reactions, and explain how each affects the rate. 9 I know the law of conservation of mass, and can explain how it relates to chemical reactions and balanced chemical equations. 10 I know the law of conservation of energy, and can explain how it relates to chemical reactions. Before After SPECTRA OF SCIENCE Chapter 6 Study Guide **Due on: _______________________** Directions: Complete each of the items below on a separate sheet of notebook paper. Be sure to number each item. 1. List 6 signs that a chemical reaction has occurred. 2. Explain what happens to bonds and atoms during a chemical reaction. 3. Label the equation below using these words: reactants, products, “yield”. a. 2CH4 + 4O2 2CO2 + 4H2O 4. Describe the difference between an exothermic and an endothermic reaction. 5. Determine which of the five main types of reactions are shown by the equations listed below: a. 2Na + Cl2 2NaCl b. Pb(NO3)2 + K2CrO4 PbCrO4 + 2KNO3 c. 2H2O 2H2 + O2 d. 3CuCl2 + 2Al 2AlCl3 + 3Cu e. Zn + 2HCl ZnCl2 + H2 f. S8 + 8O2 8SO2 + heat g. 2CH4 + 4O2 2CO2 + 4H2O 6. Identify which elements are oxidized and which are reduced in the redox reactions below. a. 3CuCl2 + 2Al 2AlCl3 + 3Cu b. Zn + 2HCl ZnCl2 + H2 7. List five factors that affect the rate of a chemical reaction. 8. Explain how each of these factors you listed in #7 will affect the reaction rate (slower or faster). 9. State the law of conservation of mass and the law of conservation of energy. 10. How do these laws relate to chemical reactions? 11. Balance the equations shown below. a. NaHCO3 H2O + CO2 + Na2CO3 c. KOH + HCl KCl + H2O b. Fe + Cl2 FeCl3 d. Cu + AgNO3 Cu(NO3)2 + Ag 12. Define these vocabulary terms: a. Radical c. Chemical energy b. Equilibrium d. Enzyme