Assignment
advertisement

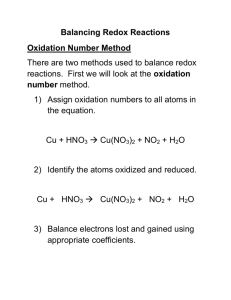
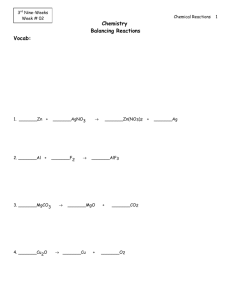
Problems: 1. Look at the following statement! 1. Oxidation is a process in which of oxygen atom is loss from compound. 2. Reduction is process in which electron is loss from compound 3. Reduction is decrease of oxidation number. 4. Oxidation form cation and anion 5. Reduction to form anode and oxide The correct statement shown by number…. 2. The highest oxidation of Cl is found in…. A. calcium chloride D. diclorin trioxides B. sodium chloride E. barium hypochlorite C. potassium per chlorite 3. Oxydator and product of oxidation from this reaction is…. 3HgS + 2HNO3 + 6 HCl → HgCl2 + S + NO + H2O A. Hg S and S D. HNO3 and Hg Cl B. HNO3 and S E. H Cl and S C. HNO3 and NO 4. Cl element which has oxidation number same as oxidation number of N in the HNO3 is.... a. KClO4 d. Cl2O7 b. KClO3 e. Cl2O5 c. KClO2 5. Look at the following reaction: 3I2 + 6KOH → 5KI + KIO3 + 3H2O The oxidator and reductor of the reaction, in a sequence is/are.... 6. There are some reaction equation: 1. Zn + 2HCl → ZnCl2 + H2 2. 2 H2 + O2 → 2H2O 3. H2 + 2Na → 2NaH 4. 2H2O2 → 2H2O + O2 Hydrogen atom with constant oxidation number is happened in the reaction number…. 7. Balance the following equation using the half-reaction methods Na + Br2 NaBr Zn + S ZnS Ag + Cr2O72- + H+ Ag+ + Cr3+ + H2O 8. Determine the kind of reaction (redox/non redox)! a. Zn + 2HCl ZnCl2 + 2HCl + S b. 2FeCl3 + H2S FeCl2 + 2HCl + S c. CaCO3 + 2HCl CaCl2 + H2O + CO2 d. 2S + 3O2 2SO3 e. SnCl2 + 2HgCl2 SnCl4 + Hg2Cl2 9. Balance the following reactions using either the oxidation number method or the half-reaction method. a. NO + As + H2O → N2O + HAsO2 b. Ce4+ + I- + OH- → Ce3+ + IO3- + H2O 10. The net equation for a given galvanic cell is: Sn(s) + 2 Ag+(aq) → Sn2+(aq) + 2 Ag(s) a. Write the two half-reactions involved, and identify each in terms of (1) site of oxidation or reduction and (2) anode or cathode. b. b. Calculate the net potential of the cell (the voltage), assuming standard conditions. c. c. Draw a fully labeled diagram of the electrochemical cell. Be sure to indicate the flow of electrons in the external circuit (through the wire and light bulb/volt meter/electrical device) and the flow of ions in the solution.