Day 3: Chemical Reactions
advertisement

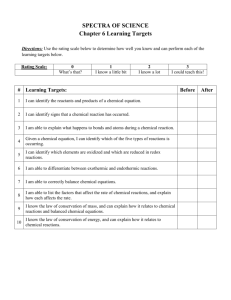
Intensive Chemistry Day 3: Chemical Reactions Katy Johanesen Ph.D. Candidate, USC Department of Earth Sciences Chemical reactions • A process where original chemical substances (reactants) transform to new chemical substances (products) Reactant 1 + reactant 2 ↔ product A + product B • Chemical reactions are often written as chemical equations (using element symbols from the periodic table) HCl + NaOH ↔ NaCl + H2O Chemical reactions • New substances often have different properties than the original substances • HCl is sour acid and can dissolve metals, NaOH is bitter base; NaCl is salty in taste and unreactive • Substances can be solid (s), liquid(l), or gas(g) • Sometimes we represent this with letters in parentheses • Zn (s) + 2HCl (aq) = ZnCl2 (s) + H2 (g) • Substances in water solution are noted (aq) • Reactions can be reversible • 2H2O ↔ 2H2 + O2 • With electricity, water can convert to hydrogen and oxygen gas • With fire, hydrogen and oxygen gas can form water Charge balancing • During all chemical reactions, electric charges must balance to equal 0 net charge • Zn + 2HCl ZnCl2 + H2 • Every reactant and product has 0 charge • While H is 1+, Cl is 1-, HCl = 0 charge • Let’s try it. Don’t worry about balancing the number or atoms yet HF (aq) + SiO2 (s) Si?F ? (g)+ H2O (l) Conservation of mass • Mass in = mass out. • no new matter is created or destroyed • Volumes and densities of reactants and products may change, but masses on both sides of the reaction are always equal • • In other words, you should have the same number of each type of atom on both sides of the equation Gases tend to leave the area of reactions, making it look like matter disappears _HF (aq) + _SiO2 (s) _SiF 4 (g) + _H2O (l) Conservation of mass • Mass in = mass out. • no new matter is created or destroyed • Volumes and densities of reactants and products may change, but masses on both sides of the reaction are always equal • • In other words, you should have the same number of each type of atom on both sides of the equation Gases tend to leave the area of reactions, making it look like matter disappears 4 HF (aq) + SiO2 (s) SiF 4 (g) + 2H2O (l) Breaking down a reaction equation • We write full reaction equations for simplicity: • Zn (s) + 2HCl (aq) = ZnCl2 (s) + H2 (g) • But in reality, we form ions in water first: • Hydrochloric acid is made by dissolving hydrogen chloride gas in water: • HCl (g) + H2O (l) = H3O+ (aq) (simplified as H+) + Cl- (aq) • A more detailed equation could be stated as: • Zn + 2H3O+ + 2Cl- = ZnCl2 + H2 + 2H2O • Products and reactants do not = 0 charge, but helps to explain what is happening (water is not usually written in the equation, it is implied it is there by the (aq) ) Half-reaction equations • Some materials can change electric charge in reactions • Zinc metal (all metals) has zero charge (Zn0) • When acids dissolve metals, the electrons from the metals transfer to cancel out positive charge (H+) from acids • Zn0 + 2H+ = Zn2+ + H2 0 (remember: 0 – [-1] = +1) • This can be written has 2 “half-reactions”: • Zn0 - 2e- = Zn2+ • 2H+ + 2e- = H2 0 • We do not usually see electrons in a final equation because they do not have a charge of 0 – and they ALWAYS cancel out: • [Zn0 - 2e-] + [2H+ + 2e-] = Zn2+ + H2 0 Simplify your life… • You can see how complex a chemical reaction really is: [Zn0 - 2e-] + [2H3O+ + 2e-] + 2Cl- = ZnCl2 + H2 + 2H2O Zn + 2HCl = ZnCl2 + H2 What’s going on in this reaction? http://www.youtube.com/watch?v=OXxyrcPdmpA _CaCO3(s) +_HCl(aq) → _CaCl _?(aq)2(aq) + _CO + _CO _H +2_H O(l)2O(l) 2(g) + 2(g) Energy in chemical reactions • Reactions may be spontaneous or may involve the input of energy (activation energy) • Combustion of gasoline to CO2 needs a spark plug • Spontaneous reactions don’t require activation energy • Exothermic vs. endothermic – Exothermic = reaction that releases energy in the form of heat (system gets hotter) – Endothermic = reaction that absorbs energy (heat) (system gets colder) Bond energy • Breaking bonds in chemical compounds requires energy – When you break the bond, energy is absorbed – When you form a bond, energy is released – The amount of energy it takes to break that bond is exactly the same as the amount of energy released when that bond is formed. This value is called the bond energy. Different bonds have different energies. The Thermite Reaction Fe2O3 + 2Al ↔ Al2O3 + 2Fe + heat Exothermic Enough heat to melt the Fe metal! Also requires high activation energy http://www.youtube.com/watch?v=o8gapa8ibK0 • The reverse of this reaction is why we should recycle It takes large amounts of heat to break bonds with Al Exothermic Reaction • K(s) + H2O(l) KOH(aq) + H2 (g)+ heat • H2 is released as a gas, so remaining solution becomes a base (depleted in H+ ions) http://www.youtube.com/watch?v=hiueYVhFTlk&feature=related • Phenolphthalein turns pink in bases • H2 is flammable! • http://www.youtube.com/watch?v=uixxJtJPVXk (a good video for alkali metal reactivity) Endothermic Reaction • Citric Acid + Sodium Bicarbonate H3C6H5O7(aq) + 3 NaHCO3(s) + heat → 3 CO2(g) + 3H2O(l) + Na3C6H5O7(aq) A more comlicated, but visually appealing endothermic reaction: http://www.youtube.com/watch?v=MyAzjSdc3Fc Is this reaction spontaneous, or does it have an activation energy? Why? • Energy of bonds broken ≠ Energy of new bonds formed (enthalpy) IF energy of bonds broken > energy of new bonds formed What happens? IF energy of bonds broken < energy of new bonds formed What happens? Bond Energy Single Bond Energies (kJ/mol) at 25°C H C N O S F Cl H C N O S F Cl Br I 436 414 389 464 339 565 431 368 297 347 293 351 259 485 331 276 238 159 222 — 272 201 243 — 138 — 184 205 201 201 226 285 255 213 — 153 255 255 — 243 218 209 193 180 Br I http://chemistry.about.com/od/chartstables/a/bondenergytable.htm 151 Endo- or Exothermic? H2 (g) + Cl2 (g) → 2 HCl (g) What is the change in Enthalpy? Red cabbage as a pH indicator • Contains flavin, an anthocyanin group compound • Chemical bonds are H+ and OH- sensitive If you add and subtract H+ and OH-, you change the bonding configuration in the molecule to absorb different colors of light anthocyanin group http://www.erowid.org/archive/rhodium/chemistry/equipment/pictures/ph-cabbage.jpg Thank You • Please email me with questions! johanese@usc.edu • I can help with Chemistry, Earth Science, or Physics.