Mole * Mass Stoichiomerty
advertisement

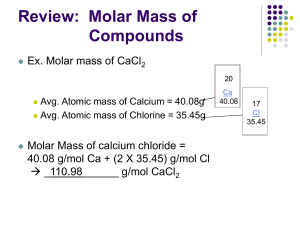
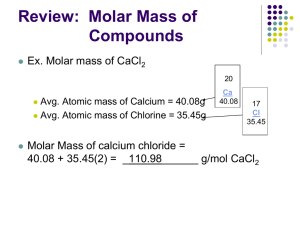
Mole – Mass Stoichiometry In a mole-mass problem, you are given the mass or moles of one substance and are asked to find the mass or moles of another substance. Solving Mole- Mass Problems Convert the given mass to moles using molar mass of given substance. Given mass of substance “A” molar mass of substance “A” = Mol “A” STEP 1 – Mass Mole Determine the number of moles for the unknown by using molar ratio of the coefficients in the balanced equation. Mol “A” x molar ratio “B” = Mol “B” “A” STEP 2 – Mole to Mole Determine the number of moles for the unknown by using molar ratio of the coefficients in the balanced equation. Mol “A” x molar ratio “B” = Mol “B” “A” STEP 1 – Mole to Mole Convert the number of moles of the unknown to mass by using molar mass of unknown substance. Mol “B” x molar mass of substance “B” = Mass “B” STEP 2 – Mole Mass 1. In a reaction between the elements aluminum and chlorine, aluminum chloride is produced. 2Al + 3Cl2 2AlCl3 a. How many grams of AlCl3 will be produced if 2.50 moles of Al react? Practice Problems (mol – g) b. How many moles of Cl2 must react to produce 12.3 g of AlCl3? 2Al + 3Cl2 2AlCl3 (g – mol) c. How many grams of aluminum will react with 3.4 moles of chlorine? 2Al + 3Cl2 2AlCl3 (mol – g) d. If 17 grams of aluminum react, how many moles of aluminum chloride will be produced? 2Al + 3Cl2 2AlCl3 (g – mol)