Balancing Redox Equations Handout
advertisement
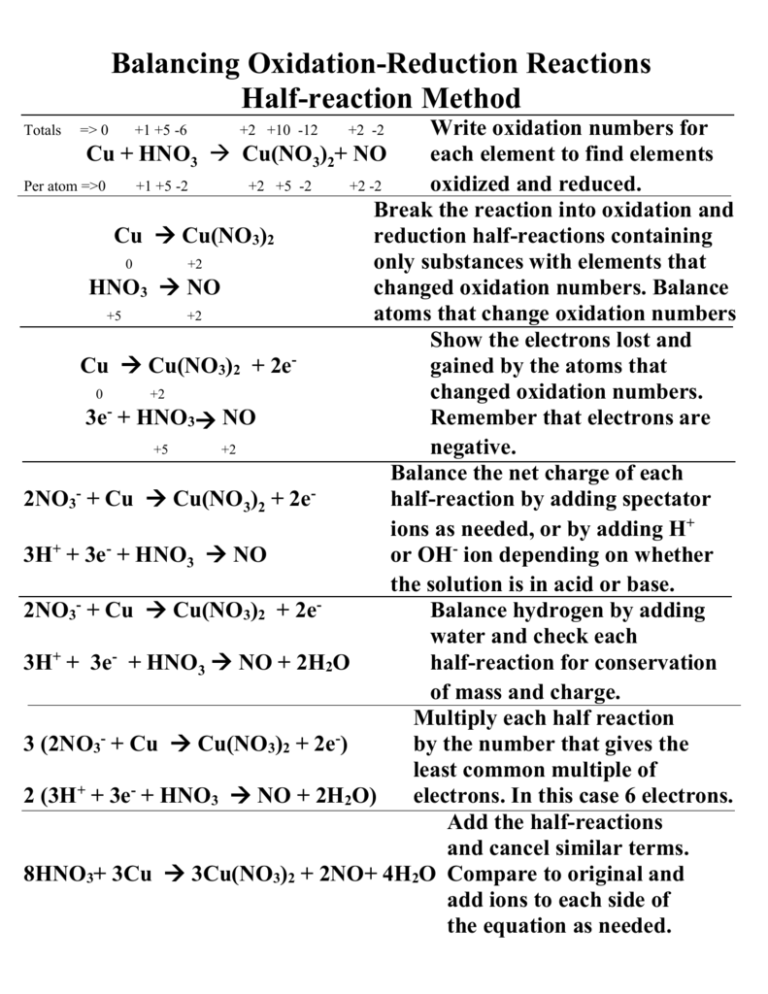
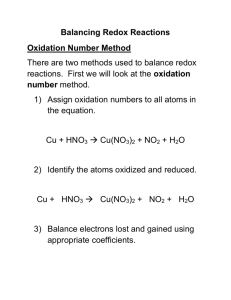
Balancing Oxidation-Reduction Reactions Half-reaction Method Write oxidation numbers for Cu + HNO3 Cu(NO3)2+ NO each element to find elements Per atom =>0 +1 +5 -2 +2 +5 -2 +2 -2 oxidized and reduced. Break the reaction into oxidation and Cu Cu(NO3)2 reduction half-reactions containing 0 +2 only substances with elements that HNO3 NO changed oxidation numbers. Balance +5 +2 atoms that change oxidation numbers Show the electrons lost and Cu Cu(NO3)2 + 2e gained by the atoms that 0 +2 changed oxidation numbers. 3e- + HNO3 NO Remember that electrons are +5 +2 negative. Balance the net charge of each 2NO3- + Cu Cu(NO3)2 + 2ehalf-reaction by adding spectator ions as needed, or by adding H+ 3H+ + 3e- + HNO3 NO or OH- ion depending on whether the solution is in acid or base. 2NO3- + Cu Cu(NO3)2 + 2eBalance hydrogen by adding water and check each + 3H + 3e + HNO3 NO + 2H2O half-reaction for conservation of mass and charge. Multiply each half reaction 3 (2NO3- + Cu Cu(NO3)2 + 2e-) by the number that gives the least common multiple of 2 (3H+ + 3e- + HNO3 NO + 2H2O) electrons. In this case 6 electrons. Add the half-reactions and cancel similar terms. 8HNO3+ 3Cu 3Cu(NO3)2 + 2NO+ 4H2O Compare to original and add ions to each side of the equation as needed. Totals => 0 +1 +5 -6 +2 +10 -12 +2 -2 Balancing Oxidation-Reduction Reactions The Oliver Method Write oxidation numbers for Cu + HNO3 Cu(NO3)2+ NO each element to find the elements Per atom=>0 +1 +5 -2 +2 +5 -2 +2 -2 that are oxidized and reduced. Rewrite the reaction to include only those atoms, ions or molecules that contain the elements that Cu + NO3- Cu+2 + NO changed oxidation numbers. Per atom=> 0 +5 +2 +2 Do not include spectator ions! IMPORTANT: Balance the atoms that change oxidation numbers. Cu +NO3- Cu+2 + NO Determine the total number of Per atom=> 0 +5 +2 +2 electrons lost and gained. Totals => 0 +1 +5 -6 +2 +10 -12 +2 -2 Lost 2eGained 3e- Balance the total number of 3Cu +2NO3 3Cu + 2NO electrons lost and gained by 0 5 +2 +2 multiplying the oxidation and 3(2e lost) reduction processes by the number 2(3e gained) that gives the least common multiple of electrons lost and gained. Balance the net charge on each side 8H+ + 3Cu + 2NO3- 3Cu+2+ 2NO of the equation by adding H+ or OHdepending upon whether the Net charge=-2 Net charge=+6 solution is acidic or basic. - +2 - - Balance hydrogens by 8H +3Cu + 2NO3 3Cu + 2NO + 4H2O adding water. This gives a balanced net ionic equation. Check oxygens to be sure. For a molecular equations 8HNO3+ 3Cu 3Cu(NO3)2 + 2NO + 4H2O add spectator ions as needed. + - +2