Activity Series Power point
advertisement

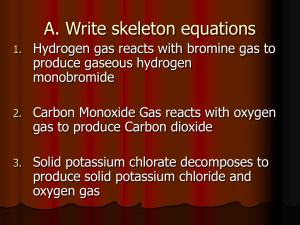
Activity Series •The ability of an element to react is the element’s activity. •The more readily an element reacts with other substances, the greater its activity is. •An activity series is a list of elements organized according to the ease with which the elements undergo certain chemical reactions. •For metals, greater activity means greater ease of loss of electrons, to form positive ions. •For nonmetals, greater activity means greater ease of gain of electrons, to form negative ions. •The order in which the elements are listed is usually determined by single-displacement reactions. 2Al (s) + 3Pb(NO3)2 (aq) → 3Pb (s) + 2Al(NO3)3 (aq) Single-displacement – one elements replaces a similar element in a compound. •The most-active element is placed at the top in the series. •It can replace each of the elements below it from a compound in a singledisplacement reaction. •An element farther down can replace any element below it but not any above it. Li can replace any element below it. Rb can replace any element below it but cannot replace Li. •Activity series are used to predict whether certain chemical reactions will occur. •Activity series are based on experimentation. Examples: According to the activity series for metals, aluminum replaces zinc. The following reaction does occur. 2Al (s) + 3ZnCl2 (aq) → 3Zn (s) + 2AlCl3 (aq) Aluminum is higher on series than zinc. Cobalt cannot replace sodium. Therefore, Co (s) + 2NaCl (aq) → no reaction Problems: Using the activity series, predict whether each of the possible reactions listed below will occur. For the reactions that occur, predict the products. 50 Zn (s) + H2O (l) → no reaction Sn (s) + O2 (g) → SnO2 Cd (s) + Pb(NO3)2 (aq) → Pb (s) + Cd(NO3)2 (aq) Cu(s) + HCl (aq)→ no reaction Cr(s) + H2O(l) → no reaction Pt(s) + O2(g) → no reaction Cd(s) + 2HBr(aq) → CdBr2(aq) + H2(g) Mg (s) + steam → Mg(OH)2(aq) + H2(g) o Br2(l) + KI(aq) → 2KBr(aq) + I2(s) Mg(s) + Co(NO3)2 (aq) → Mg(NO3)2(aq) + Co(s) Au(s) + HCl(aq) → no reaction Cu(s) + FeSO4(aq) → no reaction