The Microscale Reduction of Cyclohexanone to Cyclohexanol
advertisement

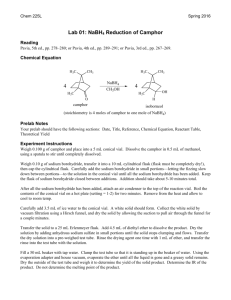
The Microscale Reduction of Cyclohexanone to Cyclohexanol As the name implies, microscale reactions are carried out on a small scale, although the actual amounts generally range from tens to hundreds of milligrams, not micrograms. There are several advantages in learning microscale techniques: many research projects involve manipulation of extremely expensive natural products which may be available in quantities of 0.5 grams or less. If several experiments are to be run on the sample, then very small amounts must be used per experiment. Also, microscale reactions generate much less waste and are generally safer to run than multigram or kilogram scale reactions. Specialized glassware (microscale lab ware) is generally used in these types of reactions. The small size reduces the amount of material lost to the sides of the vessels and allows for the manipulation and purification of milligram quantities of solids and liquids. Examples of some of this type of glassware are shown in Figure 1. Figure 1 In this experiment, ~100mg of cyclohexanone will be reduced to cyclohexanol by the action of sodium borohydride (NaBH4). Sodium borohydride is classified as a complex metal hydride in which hydrogen (as H-) can be envisioned as attacking the electrophilic carbonyl carbon. Since there are 4 moles of hydride for each mole of sodium borohydride, in principle 4 moles of ketone can be reduced with one mole of BH4-. Note that the end result is the addition of two hydrogen atoms across the carbonyl bond; however, only one of the hydrogens comes from the borohydride reducing agent. The other hydrogen comes from the protic solvent. In reactions in which no protic solvent is used (for example, with LiAlH4), the second hydrogen would be introduced on workup with water and dilute acid. Page 1 of 3 Reagents Data: Look up the relevant data for all reagents and solvents used in this lab exercise. Include the data in your “reagents” table, which should be located prior to the procedure in your report. Procedure: 1. Using a micropipette, place 100 l (0.1mL) cyclohexanone in a dry, previously weighed (tared) 5mL reaction vial equipped with an air condenser. 2. Add 250 L (0.25mL) methanol to the vial and agitate until a homogeneous solution results. 3. Add 300 L of the sodium borohydride stock solution dropwise through the top of the air condenser. The addition should take ~ 1- 2 minutes. Record any observations. ( the stock solution is prepared using the following ratio: 50mg NaOCH3, 2.5 mL methanol, 100mg NaBH4. The solution provides ~ 0.040mg NaBH4 per L solution) 4. Allow the reaction mixture to stand at room temperature with occasional agitation for 15 minutes. 5. Slowly add 1 mL of 1M HCl dropwise via a Pasteur pipet. Record any observations 6. Using a pipet, add 0.5 mL methylene chloride to the crude reaction mixture. Cap the vial and shake to thoroughly mix the two phases. Allow the phases to settle. 7. Carefully remove the methylene chloride using a Pasteur filter pipet ( a pipet fitted with a small cotton plug at the end of the tip). Transfer the material to another Pasteur filter pipet containing ~ 500mg anhydrous sodium sulfate which is clamped over a small, tared reaction vial. Allow the solution to drain into the vial. Use gentle pressure from a pipet bulb if the flow is too slow. 8. Repeat steps 6 and 7 two additional times. 9. After the last methylene chloride extract is collected in the vial, wash the sodium sulfate with 0.5mL methylene chloride and collect the washings in the same vial. 10. Under a snorkel hood, remove the methylene chloride by gentle heating in a bead bath. Use a small spatula to occasionally agitate the solution to avoid bumping. 11. When no more methylene chloride appears to be evaporating (the volume remains constant for several minutes), weigh the liquid remaining. 12. Analyze your product by IR and GC. 13. Calculate your per cent yield based on the mass of your product and GC analysis of the purity. Page 2 of 3 At minimum, your conclusion should address the following: • Did the reaction produce the expected product? What evidence do you have? • How successful was the overall process in terms of yield and purity? • Are the GC and IR analyses consistent with each other? • What challenges does this method (microscale) present vs. standard, multigram methods? Page 3 of 3