Part 1 – Simple Ionic Compounds
advertisement

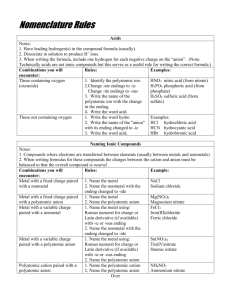
Bell Ringer Part 1 – Simple Ionic Compounds Naming Binary Ionic Compounds - Alkali (Group 1A) and Alkaline Earth Metals (Group 2A) - Name elements in the order they appear in the chemical formula. - Format: Metal Cation (+) Normal Element Name - Example: Nonmetal Anion (-) Stem of Element Name + suffix –ide NaCl Sodium Chloride (“Chlor” + -ide) - Note that the number of ions/atoms in the formula plays no part in the name for Ionic Compounds Name to the Formula – - You must write out the Ions (Na+ Cl -) and do the Criss Cross Method Part 2 – Transition Metals Naming Binary Ionic Compounds - Transition Metals (multiple charges/oxidation #’s) - Works the same way as the simple Ionic Compounds EXCEPT: o Parenthesis surrounding the transition metal’s charge/oxidation # in Roman Numerals is included. - Format: Metal Cation (+) Normal Element Name - Example: Iron Copper (Metal’s Charge) Roman Numeral (III) (II) Nonmetal Anion (-) Stem of Element Name + suffix –ide Oxide Sulfide - You must use the Anion’s (nonmetal) charge (it never changes) & the Criss Cross Method to determine the transition metal’s charge (the Roman Numerals value). o Example: Fe2O3 Fe? + O2CuS Cu? + S2- Formula was REDUCED from Cu2S2 o For 1:1 ratios the charge on the metal/cation has to be equivalent to the charge on the nonmetal/anion Name to Formula – - Same as before (Roman Numeral gives you the transition metal’s charge) Part 3 – Polyatomic Ions Naming Binary Ionic Compounds - Polyatomic Ions - Works the same way as the simple Ionic Compounds EXCEPT: o You use the given name for the Polyatomic Ion (no changes) o Include charge (roman numerals) when a transition metal is used. - Format: Metal Cation (+) (Metal’s Charge) Normal Element or Roman Polyatomic Ion Name Numeral Polyatomic Ion Anion (-) Polyatomic Ion Name o NH4+ (Ammonium) and H3O+ (Hydronium) are the only polyatomic cations you know - they will come at the beginning of a chemical formula. o Polyatomic Ions mostly end in –ate or –ite. - Example: Ca3(PO4)2 Calcium Phosphate Cu(C2H3O2)2 Copper (II) Acetate [Transition Metal] (NH4)(NO3) Ammonium Nitrate - Like before you must use the Polyatomic Anion’s charge (it never changes) & the Criss Cross Method to determine a transition metal’s charge (the Roman Numerals value). Name to Formula – - Same as before – Write out the Ions for [Na+ (NO3-)] and do the Criss Cross Method to determine the formula. Remember the Polyatomic Ions stay together – they act a single unit.