Naming Compounds Flowchart
advertisement

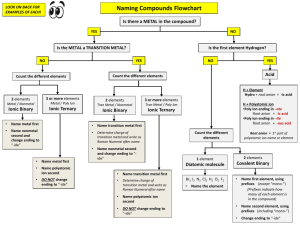
Naming Compounds Flowchart LOOK ON BACK FOR EXAMPLES OF EACH! Is there a METAL in the compound? YES NO Is the METAL a TRANSITION METAL? NO Is the first element Hydrogen? YES 3 or more elements Metal / Nonmetal Metal / Poly Ion Ionic Binary Ionic Ternary 2 elements 3 or more elements Tran Metal / Nonmetal Tran Metal / Poly Ion Ionic Binary Ionic Ternary Name metal first • Name transition metal first • Name nonmetal second and change ending to “-ide” • Determine charge of transition metal and write as Roman Numeral after name • Name nonmetal second and change ending to “ide” Name metal first • Name polyatomic ion second • DO NOT change ending to “-ide” Acid H + Element Hydro + root anion + -ic acid • • YES Count the different elements Count the different elements 2 elements NO • Name transition metal first • Determine charge of transition metal and write as Roman Numeral after name • Name polyatomic ion second • DO NOT change ending to “-ide” H + Polyatomic ion •Poly ion ending in –ate Root anion + -ic acid •Poly ion ending in –ite Root anion + -ous acid Count the different elements 1 element Root anion = 1st part of polyatomic ion name or element 2 elements Diatomic molecule Covalent Binary Br2 I2 N2 Cl2 H2 O2 F2 • Name first element, using prefixes (except “mono-”) (Prefixes indicate how many of each element is in the compound) • Name second element, using prefixes (including “mono-”) • Change ending to “-ide” • Name the element ROMAN NUMERALS I one II two III three IV four V five DETERMINING THE CHARGE OF A TRANSITION METAL 1. PREFIXES Find the total charge of the Anion Anion Oxidation # x Anion Subscript 2. Covalent Compounds ONLY: Look at the subscripts to tell you how many of each element you have Determine charge of Transition Metal Total Anion charge 3. ÷ One Two Three Four Five Six Seven Eight Nine Ten Metal subscript Transition metal charge is always positive (+) Example: Polyatomic Ion List Step 1: Step 2: Step 3: -2 x 3 = -6 -6 ÷ 2 = -3 +3 Charge of Fe monoditritetrapentahexaheptaoctanonadeca- Answer: Iron III oxide COVALENT COMPOUNDS EXAMPLES IONIC COMPOUNDS EXAMPLES Nonmetal/Nonmetal Metal/Nonmetal P2O5 __________________________ KCl __________________________ CCl4 __________________________ Al2O3 __________________________ Metal/Polyatomic Ion Metal/Polyatomic Ion Mg(NO3)2 __________________________ NaC2H3O2 __________________________ Cl2 __________________________ Br2 __________________________ ACID EXAMPLES Transition Metal/Nonmetal Fe2O3 __________________________ CuCl2 __________________________ H + Element • HCl ________________________________ • H3P ________________________________ H + Polyatomic Ion Transition Metal/Polyatomic Ion CuCrO4 __________________________ Zn(OH)2 __________________________ • H2SO4 ________________________________ • H2SO3 ________________________________ • H3PO4 ________________________________