Basic Inorganic Nomenclature
advertisement

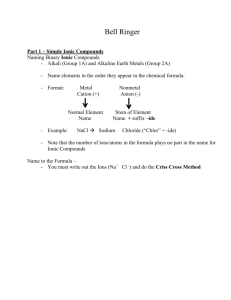
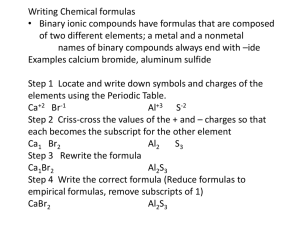
Basic Inorganic Nomenclature Compiled by Josh Schoenly, from: Mr. Richard Hallowell’s Chemistry lecture notes (1999-2000) Quick Study Academic. “Chemistry.” Bar Charts Inc: May 2001. ACDLabs Freeware 5.0; 3D viewer, ChemSketch. Wilbraham, Antony C., Dennis D. Staley, Candace J. Simpson, Michael S. Matta. Chemistry: Second Edition. Addison Wesley Publishing: Menlo Park, Calif. 1990. Introduction – One of the more tedious and less interesting topics about the science of Chemistry among students would be nomenclature. While for most of the time, a student of chemistry can simply determine an answer via stoichiometry, VSEPR theory and a general good logical sense of reactions and electron distribution; the naming of compounds may come as an un-welcomed guest. Most students will simply memorize names, not even give a second glance to the logic behind the nomenclature. The skill of naming compounds, however, can be an important aide when trying to determine the structure of a compound. This skill can also be learned without the tediousness of creating flashcards and memorizing names of different compounds. IUPAC, International Union of Pure and Applied Chemistry, has created a logical set of instructions for the naming of compounds so as to create a universal language among chemists. The student of chemistry is therefore left with a set of instructions as well as Latin prefixes and suffixes in order to name compounds fairly easily. The purpose of this paper is to introduce the new student of chemistry to the art of basic inorganic nomenclature in order to create a sense of stability and order for the student in his / her journey through world of chemistry. I. Binary Compounds – Compounds that consist of only 2 elements A. Metal and a nonmetal (ionic bond) 1. Molecular Formula Name a. Name the metal b. Name the nonmetal c. Add the prefix (-ide) to the end of the nonmetal d. Examples: (1). NaCl – Sodium Chloride (2). AlP – Aluminum Phosphide (3). BaBr2 – Barium Bromide (4). Li2O – Lithium Oxide (5). Rb2Se – Rubidium Selenide 2. Name Molecular Formula a. Determine the charge on the metal using a Periodic Table b. Determine the charge on the nonmetal using a Periodic Table c. If charges are equal, write down the two elements as is. d. If the charges are unequal, balance using subscripts. e. Examples: (1). Lithium Fluoride Li and F Li is an alkali metal and therefore has a charge of +1 Li+1 F is a nonmetal, Halide (Halogen anion) and has a charge of –1 F-1 Both charges are equal so: LiF (2). Cesium Selenide Cs and Se Cs is an alkali metal and has a charge of +1 Cs+1 Se is a group 7 metal and has a charge of –2 Se-2 Charges are not equal so Cs must have a subscript of 2: Cs2Se : Since subscripts are multiplied by the charge to get a balance. (3). Aluminum Oxide Al and O Al is a group 3 metal and has a charge of +3 Al+3 O is a group 6 nonmetal and has a charge of –2 O-2 Charges are not equal so Al must have a subscript of 2 and O a subscript of 3 to balance the molecular equation: Al2O3 B. Two Nonmetals (covalent bond) 1. Molecular Formula Name a. Use a prefix on the first element if the first element has more than 1 atom b. Name the first element c. Always use a prefix for the second element d. Name the second element e. Add the suffix (-ide) to the end of the second element Prefix Number of atoms Mono1 Di2 Tri3 Tetra4 Penta5 Hexa6 Hepta7 Octa8 Nona9 Deca10 Undeca11 Dodeca12 f. Examples: (1). PCl3 – Phosphorus trichloride (2). N2O5 – Dinitrogen pentoxide (3). N2O – Dinitrogen monoxide (4). CCl4 – Carbon tetrachloride 2. Name Molecular Formula a. Determine the symbols for the two elements b. Use prefixes to put in subscripts to denote number of atoms c. Examples (1). Carbon dioxide – CO2 (2). Sulfur hexachloride – SCl6 C. Naming compounds with transition metals 1. Molecular Formula Name (classical system) a. Determine the charge on the metal (1). Trick – Look at the charge on the anion (2). Trick – Look at the subscripts b. Name the metal using the classical system (below table) (1). Metals of lower charge will have the suffix (-ous) (2). Metals of a higher charge will have the suffix (-ic) c. Name the nonmetal as seen in rule IA. Element (English) Charge Latin root Stock Classical Iron (Fe) +2 Ferrum Iron (II) Ferrous Iron (Fe) +3 Ferrum Iron (III) Ferric Tin (Sn) +2 Stannum Tin (II) Stannous Tin (Sn) +4 Stannum Tin (IV) Stannic Lead (Pb) +2 Plumbum Lead (II) Plumbous Lead (Pb) +4 Plumbum Lead (IV) Plumbic Copper (Cu) +1 Cuprum Copper (I) Cuprous Copper (Cu) +2 Cuprum Copper (II) Cupric +2 Mercury (Hg2 ) +1 Mercurius Mercury (I) Mercurous Mercury (Hg+2) +2 Mercurius Mercury (II) Mercuric Chromium (Cr) +2 Chromium (II) Chromous Chromium (Cr) +3 Chromium (III) Chromic Manganese (Mn) +2 Mangnes Manganese (II) Manganous Manganese (Mn) +3 Mangnes Manganese (III) Manganic Cobalt (Co) +2 Cobalt (II) Cobaltous Cobalt (Co) +3 Cobalt (III) Cobaltic Zinc (Zn) +2 Silver (Ag) +1 d. Examples (1). FeO – Ferrous Oxide (2). PbCl4 – Plumbic Chloride (3). Hg2Se – Mercurous Selenide (4). AgCl – Silver Chloride 2. Molecular Formula Name (stock system) a. Follow the classical system rules for determining charge on metal b. Use the “stock” column of the table above to name cation c. Name the nonmetal as seen in Rule IA. d. Examples (1). FeO – Iron (II) Oxide (2). PbCl4 – Lead (IV) Chloride (3). Hg2Se – Mercury (I) Selenide 3. Name (classical and stock system) a. Determine charge on metal using the table above (1). Charge in classical needs to be memorized (2). Charge in stock gives the charge in Roman numerals b. Balance using subscripts II. Polyatomic ions – A group of atoms that act as an ion. A. Tables of polyatomic ions. 1. Polyatomic anions: -1 Formula CH3COONO2NO3ClOClO2ClO3ClO4CNOHSCNHCO3HSO3HSO4H2PO4MnO4- Name Acetate Nitrite Nitrate Hypochlorite Chlorite Chlorate Perchlorate Cyanide Hydroxide Thiocyanate Hydrogen carbonate, bicarbonate Hydrogen sulfite, bisulfite Hydrogen sulfate, bisulfate Dihydrogen phosphate Permanganate 2. Polyatomic anions: -2 Formula CO32SO42SO32CrO42O22HPO42Cr2O72S2O32S22SiO32- Name Carbonate Sulfate Sulfite Chromate Peroxide Hydrogen phosphate, biphosphate Dichromate Thiosulfate Disulfide Silicate 3. Polyatomic anions: -3 Formula PO43PO33- Name Phosphate Phosphite 4. Polyatomic anions: -4 Formula SiO445. Polyatomic cations: +1 Formula NH4+ H3O+ Name Silicate Name Ammonium Hydronium B. Molecular Formula Name 1. Locate the common groups of polyatomic ions in the formula 2. Name the polyatomic ions 3. Name the remaining cation/anion if there was only one polyatomic ion 4. Examples a. Na3PO4 - Looking at the table, the only polyatomic ion in this formula is phoshphate. It acts as the anion. The metal in this equation is sodium, therefore, the compound is called sodium phosphate. b. Ba(NO2)2 – Looking at the table, the only polyatomic ion in this formula is nitrite. It acts as the anion. The metal in this equation is Barium, therefore, the compound is called barium nitrite. c. (NH4)2SO4 – Looking at the tables, there are two polyatomic ions in this formula. Ammonium acts as the cation while sulfate acts as the anion. The compounds name is ammonium sulfate. C. Name Molecular Formula 1. Write down the formula for any polyatomic ion using the tables on polyatomic ions as well as the charge. 2. Write down any none polyatomic ions given in the name of the formula with their charge as well. 3. Balance the equation using subscripts so that the total charge of the compound is 0. 4. Examples a. Potassium dichromate – The polyatomic ion in the equation is dichromate, which is given as: Cr2O72-. It is acting as the anion. Potassium, or K+, is acting as the cation and is monatomic. Balancing using subscripts gives K2Cr2O7. b. Ammonium chloride – The polyatomic ion in the equation is ammonium, which is given as: NH4+. It is acting as the cation. Chloride, or Cl-, is acting as the anion and is monatomic. Balancing using subscripts gives NH4Cl. c. Ammonium phosphite – The polyatomic cation in the equation is ammonium, which is given as: NH4+. The polyatomic anion in the equation is phosphite, which is given as: PO33-. Balancing using subscripts gives (NH4)3PO3. III. Acids – more specifically, naming bronsted-lowry acids. Dissolved in water. A. Binary acids – Acids with only two elements. 1. Add prefix hydro2. Name anion (since H+ is acting as the cation) 3. Add suffix –ic 4. Add word acid 5. Examples a. HCl – Hydrochloric acid b. H2S – Hydrosulfuric acid c. H3P – Hydrophosphoric acid d. HBr – Hydrobromic acid B. Acids with anions that end in –ite. 1. Name the anion (since H+ is acting as the cation) 2. Replace the suffix –ite with –ous 3. Add word acid 4. Examples a. H2SO3 – Sulfurous acid b. H3PO3 – Phophorous acid c. HNO2 – Nitrous acid C. Acids with anions that end in –ate. 1. Name the anion (since H+ is acting as the cation) 2. Replace the suffix –ate with –ic 3. Add the word acid 4. Examples a. H2SO4 – Sulfuric acid b. H3PO4 – Phophoric acid c. HNO3 – Nitric acid d. H2CO3 – Carbonic acid IV. Some Common Names Formula H2O CaO Ca(OH)2 NaOH K2CO3 NaHCO3 NaCl HCl N2O Na2S2O3 Chemical Dihydrogen monoxide, hydric acid Calcium oxide Calcium Hydroxide Sodium hydroxide Potassium carbonate Sodium bicarbonate Sodium chloride Hydrochloric acid Dinitrogen monoxide Sodium thiosulfate Common Name Water Lime Slaked lime Lye Potash Baking soda, bicarbonate soda Table salt Muriatic acid Laughing gas Hypo