Naming and Balancing Equations
advertisement

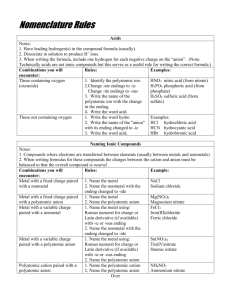
Naming and Balancing Equations Review Writing formulas for binary ionic compounds • Composed of two elements -Monatomic cation(name of element followed by the word ion) -Monatomic anion(name of element ends with -ide) • Ionic compounds are electrically neutral(net charge must be zero), therefore we must balance the charges when writing the formula • Use the element symbols and remember the cation is always written first Writing formulas example • Calcium bromide • Composed of calcium ions, Ca2+ and bromide ions, Br – • The ions must combine in a 1:2 ratio • Each calcium ion with its 2+ charge must combine with(or be balanced by) two bromide ions, each with a 1charge. • Formula for calcium bromide is CaBr2 Naming binary ionic compounds • First write the name of the cation followed by the name of the anion(ending in –ide) • When cations have more than one common charge the roman numeral must be included in brackets after the element name. Naming example • CoI3 • Cobalt and iodine • Since there are 3 iodine in the compound, for the net charge to be zero, the charge on cobalt must be 3+ • The name of the compound is cobalt(III) iodide Ternary ionic compounds • Contains atoms of three different elements • Usually contains one or more polyatomic ions • Procedure for writing the formula is same as binary compounds • First write down the symbol and charge of the ions • Then balance the charges • An –ate or –ite ending on the compound indicates it contains a polyatomic anion • Exceptions: hydroxide and cyanide Example • Calcium nitrate • Composed of calcium ions, Ca2+, and nitrate ions, NO3• To balance the charges two nitrate ions are needed to balance the 2+ charge on calcium • Parentheses are used around the nitrate ion in the formula because two nitrate ions are need • Formula is: Ca(NO3)2 Binary molecular compounds • Composed of two nonmetallic elements. • Ionic charges are not used in writing formulas • Often combine in more than one way • Prefixes are used to show how many atoms of each element are present in each molecule • The second element’s name is written with an –ide ending • Also note the vowel at the end of the prefix mono- is dropped when the name of the element begins with a vowel Writing the formula example • • • • N 2O Nitrogen and oxygen are present Two nitrogen and one oxygen Dinitrogen monoxide Writing formulas for molecular compounds • Very easy because the prefixes tell you the subscript of each element in formula • Tertaiodine nonoxide • Tetra=4 and nono=9 • I4O9 Writing formulas of Acids • Acids are compounds that give off hydrogen ions when dissolved in water. General formula for acids are HX(X is a monatomic or polyatomic anion). • Rules on naming • 1) when anion ends in –ide, and the acid name begins with the prefix hydro- the acid name is the stem of the anion ending –ic followed by acid • 2) when anion ends in –ite, the acid name is the stem of the anion ending in –ous followed by acid • 3) when anion ends in –ate , the acid name is the stem of the anion ending in –ic followed by the word acid. Example • HClO • Using rule 1- the ending of the polyatomic anion ends in –ide, therefore the acid is: hydrocyanic acid Balancing Rules • Determine the correct formula for all reactants and products and write them on the appropriate sides of reaction • Count the number of atoms of each element in the reactants and products (polyatomic ion appearing on unchanged on both sides counts as one unit) • Balance elements, other than hydrogen and oxygen, by using coefficients • Balance hydrogen and oxygen last • Check each atom and polyatomic ion to be sure equation is balanced • Finally make sure the coefficients are at the lowest possible ratio Example When hydrogen and oxygen react, the product is pure water. Write a balanced equation for reaction: • First we can write a skeleton equation because the formulas for the reactants and products are known H2(g) + O2(g) H2 O(l) • If we put a coefficient of 2 in front of H2O, the oxygen becomes balanced. H2(g) + O2(g) ) 2H2O(l) • Now there are twice as many hydrogen atoms in the product as there are in the reactants. To correct this, put a coefficient of 2 in front of H2 and equation is now balanced. 2H2(g) + O2(g) ) 2H2O(l) • Check the coefficients. They must be in their lowest possible ratio 2(H2)s 1(O2), and 2(H2O)