Chapter 5-Nomenclature
advertisement

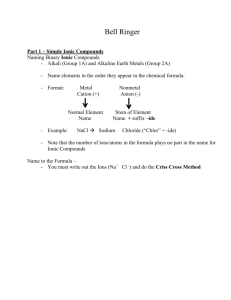
Chapter 5-Nomenclature Common Names - Exceptions • H2O = water, steam, ice • NH3 = ammonia • CH4 = methane • NaCl = table salt • C12H22O11 = table sugar Naming Starts with Classifying Compounds • Binary Compounds = only 2 elements • Compounds containing polyatomic ions • Acids = formula often starts with H Classifying Binary Compounds • Compounds containing a metal and a nonmetal are binary ionic – Type I and II • Compounds containing two nonmetals – Type III • Compounds containing H and a nonmetal = Acids Binary Ionic • Made of metal cation and nonmetal anion • Name by naming the ions Metal Cations Type I Metal Ion • Metals that can only have one possible charge • Determine charge by position on the Periodic Table • The name of the ion is the same as the name of the metal with no special designations. Type II Metal Ion • Metals that can have more than one possible charge • Determine metal cation’s charge from the charge on anion • Metals that can have two possible charges are identified by adding a roman numeral to the name of the metal. The roman numeral matches the charge of the metal ion. Type I Binary Ionic Compounds Contain Metal Cation + Nonmetal Anion Metal listed first in formula & name Name metal cation first, name nonmetal anion second Simple metal cation name is the metal name o simple metals are Groups 1A, 2A and Al, Ga & In Nonmetal anion named by changing the ending on the nonmetal name to -ide Type II Binary Ionic Compounds Contain Metal Cation + Nonmetal Anion Metal listed first in formula & name Name metal cation first, name nonmetal anion second Metal cation name is the metal name followed by a Roman Numeral in parentheses to indicate its charge o Determine charge from anion charge o Common Type II cations in Table 5.2 Nonmetal anion named by changing the ending on the nonmetal name to -ide Determining the Charge on a Cation – Au2S3 Determine the charge on the anion a. Au2S3 - the anion is S, since it is in Group 6A, its charge is -2 Determine the total negative charge a. since there are 3 S in the formula, the total negative charge is -6 Determine the total positive charge a. since the total negative charge is -6, the total positive charge is +6 Divide by the number of cations a. since there are 2 Au in the formula & the total positive charge is +6, each Au has a +3 charge Type III - Binary Compounds of 2 Nonmetals (Covalent) • Name first element in formula first, use the full name of the element • Name the second element in the formula as if it were an anion – However, remember these compounds do not contain ions! • Use a prefix in front of each name to indicate the number of atoms • Never use the prefix mono- on the first element Compounds Containing Polyatomic Ions • Polyatomic ions are charged entities that contain more than one atom – You do not need to memorize these- look up names on your ion chart! • Polyatomic compounds contain one or more polyatomic ions • Name polyatomic compounds by naming cation and anion – Non-polyatomic ions named like Type I and II • Polyatomic Acids contain H+ and a polyatomic anion Patterns for Polyatomic Ions Elements in the same column on the Periodic Table form similar polyatomic ions o same number of O’s and same charge o ClO3- = chlorate BrO3- = bromate If the polyatomic ion starts with H, add hydrogen- before the ions name and add 1 to the charge o CO32- = carbonate HCO3- = hydrogen carbonate Patterns for Polyatomic Ions- You do not need to know this- but this is good to be familiar with. • -ate ion plus 1 O same charge, per- prefix – perchlorate = ClO4• -ate ion – chlorate = ClO3• -ate ion minus 1 O same charge, -ite suffix – chlorite = ClO2• -ate ion minus 2 O same charge, hypo- prefix, -ite suffix – hypochlorite = ClOAcids • Contain H+ cation and anion • Binary acids have H+ cation and a nonmetal anion • Oxyacids have H+ cation and a polyatomic anion Writing the Formulas from the Names • For Type III compounds, use the prefixes to determine the subscripts • For Type I, Type II, polyatomic Compounds and Acids – Determine the ions present – Determine the charges on the cation and anion – Balance the charges to get the subscripts Summary: Don’t make naming more difficult that it needs to be Decide if it is a Type I, II or III Use the steps to name- BINARY COMPOUNDS ARE EASY! IONS are EASY!! LOOK UP THE NAMES!