6.3-potential
advertisement

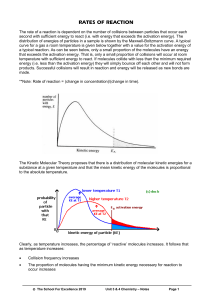
6.3 Theories of Reaction Rates Why do factors such as temperature and concentration increase or decrease the rate of a reaction? Collision theory In order for a reaction to occur, reacting particles (atoms, molecules, or ions) must collide with another. Rates will increase if there are more collisions per unit time. Increasing concentrations o Ex increased concentration puts more reactant particles in a smaller volume this will increase # of collisions. Like putting more vehicles on a stretch of highway. Increasing Surface area o o With greater surface area more reactants are exposed thus causing more collisions Starting a fire with kindling vs large boards Not every collision between reactants results in a reaction. Reaction rates vary For a collision to be effective (results in Product formation), it must satisfy both these criteria 1. Correct orientation of reactants 2. Sufficient collision energy a. Activation Energy, Ea Minimum collision energy that is required for a successful reaction. The collision energy depends on the kinetic energy of the colliding particles The difference between the initial energy of the reactants and the energy of the activated complex at the transition state b. Maxwell –Boltzmann distribution A curve showing the distribution of kinetic energy of particles at a constant temperature. At a given temperature only a certain fraction of the molecules in a sample have enough kinetic energy to react (energy equal or Greater than Ea) At increased temperatures, more particles have enough energy to react. At higher molecular speeds, there are more molecular collisions and hence a greater chance of reaction If the average molecular kinetic energy increases, more of the collisions will have enough energy to overcome the activation energy barrier. Usually an increase in temperature of 10oC will double the rate of reaction Transition State Theory Explains what happens when molecules collide in a reaction. It examines the transition from reactants to products Kinetic energy of reactants is transferred to potential energy as the reactants collide. o Kinetic energy is the energy of something moving o Potential energy is the energy stored in an object Rubber band example Potential energy diagrams For Exothermic reactions Ea(rev)= Ea(fwd) + For endothermic reactions H Ea(rev)= Ea(fwd) - H There is no way to predict Activation energy (Ea) of a reaction from its enthalpy change. Transition state The top of the activation energy barrier on a potential energy diagram Activation Complex The chemical species that exist at the transition state. ( a species that is neither product or reactant it is somewhere in between) a) Sketch a potential energy diagram for an endothermic reaction in which Ea= 80kJ and H = +50 kJ b) What would the Ea and H be for the reverse reaction. a) Sketch the potential energy diagram for a reaction in which Ea(Fwd)= 134kJ and b) Calculate Ea(rev) and H (rev). Label Ea(rev) on your diagram. Questions 14,15 page 294 2,3 page 296 BLM 6-3 H = -226 kJ