6.1 c) Collision Theory and Factors Affecting Reaction Rates
advertisement

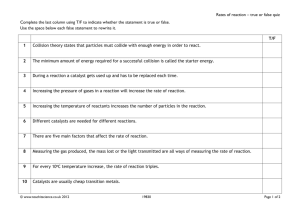
6.1 c) COLLISION THEORY AND FACTORS AFFECTING REACTION RATES Collision theory is used to explain rates of reaction. Recall: *For a reaction to occur, the bonds of the reactant molecules must be_____________. The energy required to accomplish this is provided by the _____________ (i.e. movement) energy of the molecules. POSTULATES OF COLLISION THEORY: 1. The particles of a chemical system are in constant random motion. The higher the __________ __________ __________ of the particles, the higher the ________________. 2. A chemical reaction must involve _________ of particles with each other or the walls of the container. 3. The collision must be ______________ (“successful”). For a collision to be effective, molecules must: (i) collide with _______________ energy; and, (ii) collide with correct __________________. http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/collis11.swf * The rate of a given reaction depends on two factors: (i) the _________________ of collisions; and, (ii) the fraction of those collisions that are _______________. *[rate = collision ________________ x fraction _____________] FACTORS AFFECTING REACTION RATE *Factors that INCREASE collision frequency: 1. ___________________ 2. __________ ________ *3. __________________ *Factors that INCREASE the proportion of particles that are able to collide effectively: 1. ___________________ 2.__________________ *There are four main factors that can INCREASE the rate of a chemical reaction: 1. INCREASING THE TEMPERATURE OF A CHEMICAL REACTION Higher temperatures generally accompany higher reaction rates and vice versa. E.g. Milk will sour much quicker at room temperature opposed to being in a refrigerator due to accelerated bacterial reactions. Theoretical Effect of INCREASING TEMPERATURE: *Increasing the temperature causes the shape of the Maxwell-Boltzmann distribution curve to __________ and shift to the __________. This results in a large increase in the proportion of molecules that are able to react. *N.B. A change in temperature DOES NOT alter the magnitude of the ACTIVATION ENERGY. 2. ADDITION OF A CATALYST Catalysts lower the _____________________ Energy associated with a reaction, thereby increasing the rate of reaction. *Activation Energy (Ea) the minimum energy that colliding particles need for a reaction to occur Common mistake: You do not “give the particles activation energy”. Activation energy is the energy BARRIER. You do not give runners “hurdles”, you give them the energy to jump over the hurdles. http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/activa2.swf Theoretical Effect of ADDING A CATALYST: *Catalysts provide an alternative lower energy pathway from reactant to products. If the new pathway (mechanism) has _______________ activation energy, a greater fraction of molecules possess the minimum required energy and the reaction rate increases. *A catalyst: is a substance that speeds up the rate of a reaction __________________ being consumed; is a component of the reaction _______________________; altered during a reaction; is ____________________ by the end of the reaction. *A biological catalyst is called an ____________. (E.g. lactase, lipase, amylase, pepsinogen) *Catalysts accelerate a chemical reaction by providing an alternative ______________ that has lower ________________ energy. As a result, more reactant molecules have the minimum energy to react. E.g. (MECHANISM) Step 1: A + catalyst A-catalyst Step 2: A-catalyst + B AB + catalyst (regenerated) Overall *Reaction intermediate = _____________ *TWO TYPES OF CATALYSTS: (i) ___________________ Catalysts catalysts that exist in the _________ phase as the reactants / most often catalyze gaseous and aqueous reactions Homogeneous catalysts increase the rate by decreasing the activation energy via an alternate _____________________. (i.e. an alternate “pathway” from reactants to products) Example (g) 2SO(g) + 2O2(g) 2SO3(g) NO (…the Contact process) (ii) ____________________ Catalysts catalysts that exist in a phase that is ________________ from the phase of the reaction it catalyzes Examples: (s) 2NH3(g) 3H2(g) + N2(g) Fe (…the Haber process) 2SO(g) + 2O2(g) 2SO3(g) V2 O5 ( s ) (s) alkene + H2(g) alkane Ni (s) e.g. C2H4(g) + H2(g) C2H6(g) Ni Heterogeneous catalysts ___________________ (to gather on a surface) reactant particles; that is, they bring reactant particles closer together, thereby enabling them to react more readily. ENERGY PROFILES FOR EXOTHERMIC AND ENDOTHERMIC REACTIONS *Activated Complex an unstable chemical species containing ______________ broken and _____________formed bonds representing the __________________potential energy point in the change; also known as the _______________ state. 3. INCREASING THE CONCENTRATION OF THE REACTANTS (OR PRESSURE OF A GAS) The concentration of a _____________ or the pressure of a ____ is simply the number of particles per unit volume. If the initial concentration of a reactant increases, the reaction rate generally ______________. e.g. Consider the destruction of limestone statues via acid rain (from SO2 pollution, for example): SO2(g) + H2O(l) H2SO4(aq) CaCO3(s) + H2SO4(aq) CaSO4(aq) + H2O(l) + CO2(g) The rate of carbon dioxide gas production / limestone statue degeneration is greater when the sulfuric acid is more concentrated, 1 molar versus 0.1 molar, for example. Theoretical Effect of Concentration/Pressure ____the concentration = ____ collision frequency = ___ successful collisions = ___ rate of reaction 4. DECREASING THE SIZE OF THE PARTICLES IN THE SOLID PHASE (INCREASING SURFACE AREA) Increasing the surface area of a reactant increases the reaction rate. e.g. *Kindling will burn much faster than a log of wood *If you cut a potato in half, then the boiling water can reach the “inside” surface that it couldn’t previously.) *Finely powdered CaCO3(s) (chalk) will react much quicker with HCl(aq) than non-powdered CaCO3(s). Theoretical Effect of decreasing particle size / increasing surface area: ___ particle size = ___ surface are = _____ collision frequency = ____ successful collision = ____ rate of reaction *Note that this factor only applies to ____________________reactions (i.e., reactions involving a gas or liquid with a solid. Surface area affects collision frequency because reactants can collide only at the surface where the substances are in contact. *5. NATURE OF THE REACTANT e.g. The activity series of metals (e.g. Lithium and potassium react with water while silver and gold do not.) Theoretical Effect of Nature of Reactant: *The chemical nature of reactants affects the threshold energy in two possible ways: (i) Some molecules have low ________________ energy because of ________ bonds. As a result, a larger fraction of molecules are capable of colliding successfully. (ii) Molecules with ____________ collision geometry are often ______ reactive. *Why?