Unit 2: Quantum Numbers Review Packet
advertisement

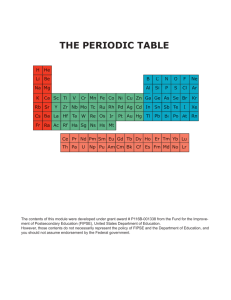
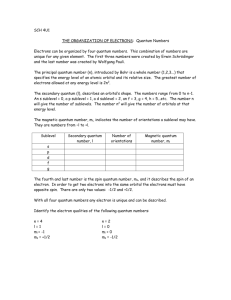
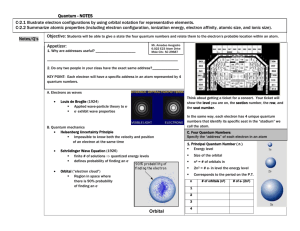
Name:___________________________ Review sheet for Quantum Numbers and e- config 1. Using your periodic table locate the element silicon, Si. a. Using the arrow diagram shown above, draw the correct orbital diagram for silicon, Si. b. Now, write the abbreviated electron configuration for silicon. c. Lastly, write the periodic table electron configuration for silicon. d. Using the orbital diagram from question 1a, write the 14 sets of quantum numbers that describe the 14 electrons of silicon. 2. Indicate the maximum number of electrons an atom can have using only certain parts of their set of four quantum numbers for each of the following. a. n = 3 b. n = 4, l = 2 c. n = 2, l = 2 d. n = 4, l = 3, m = -1, s = +1/2 e. n = 3, l = 1, m = +2 f. n = 6, l = 3, m = -3 3. Write the quantum numbers associated with each of the following. a. The fifth principle energy level b. The 6s sublevel c. An orbital on the 3d sublevel d. The first electron added to the 4f sublevel 4. Indicate the maximum number of electrons an atom can have using only certain parts of their set of four quantum numbers for each of the following. a. n = 8 b. n = 2, l = 1 c. n = 4, l = 3, m = +2 d. n = 7, l = 1, m = +2, s = -1/2 e. n = 6, l = 0, m = 0, s = +1/2 f. n = 4, l = 2, m = -3 g. n = 8, l = 2 5. With reference to quantum numbers, explain why the 4f sublevel can hold a maximum of 14 electrons. _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 6. Using your periodic table locate the element vanadium, V. a. Using the arrow diagram shown on the first page, draw the correct orbital diagram for vanadium. b. Now, write the abbreviated electron configuration for vanadium. c. Lastly, write the periodic table electron configuration for vanadium. d. Using the orbital diagram from question 6a, write the 23 sets of quantum numbers that describe the 23 electrons of vanadium. 7. Write a complete orbital diagram, electron configuration and periodic table electron configuration for zirconium, Zr. 8. Write a complete orbital diagram, electron configuration and periodic table electron configuration for germanium, Ge.