Worksheet 9
advertisement
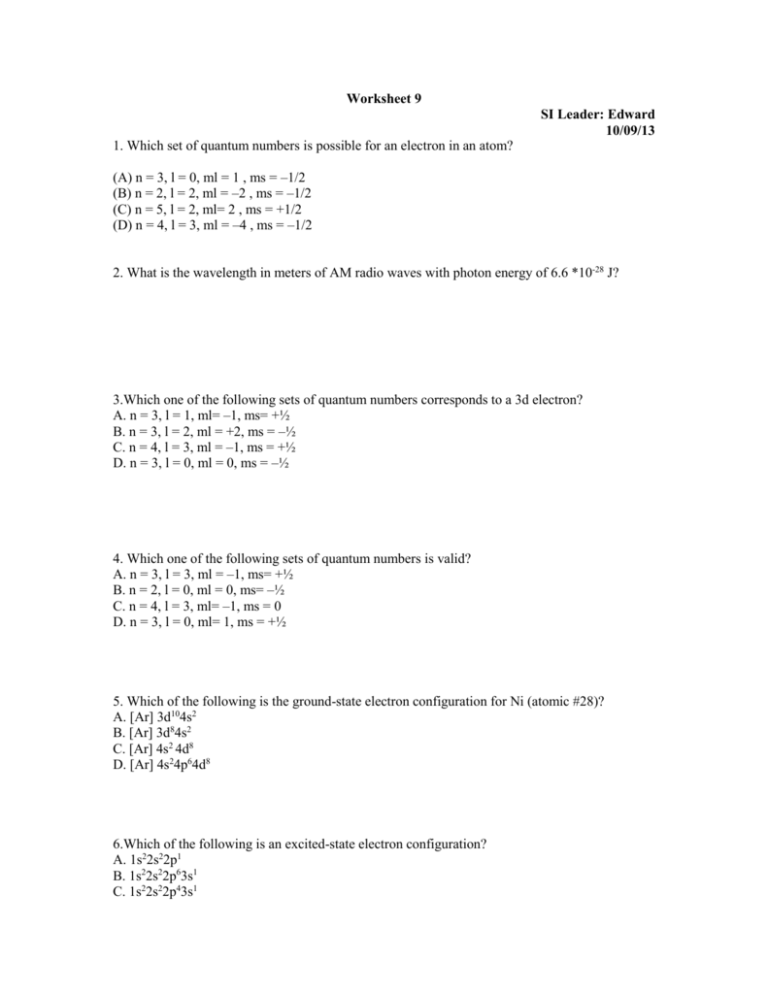
Worksheet 9 SI Leader: Edward 10/09/13 1. Which set of quantum numbers is possible for an electron in an atom? (A) n = 3, l = 0, ml = 1 , ms = –1/2 (B) n = 2, l = 2, ml = –2 , ms = –1/2 (C) n = 5, l = 2, ml= 2 , ms = +1/2 (D) n = 4, l = 3, ml = –4 , ms = –1/2 2. What is the wavelength in meters of AM radio waves with photon energy of 6.6 *10-28 J? 3.Which one of the following sets of quantum numbers corresponds to a 3d electron? A. n = 3, l = 1, ml= –1, ms= +½ B. n = 3, l = 2, ml = +2, ms = –½ C. n = 4, l = 3, ml = –1, ms = +½ D. n = 3, l = 0, ml = 0, ms = –½ 4. Which one of the following sets of quantum numbers is valid? A. n = 3, l = 3, ml = –1, ms= +½ B. n = 2, l = 0, ml = 0, ms= –½ C. n = 4, l = 3, ml= –1, ms = 0 D. n = 3, l = 0, ml= 1, ms = +½ 5. Which of the following is the ground-state electron configuration for Ni (atomic #28)? A. [Ar] 3d104s2 B. [Ar] 3d84s2 C. [Ar] 4s2 4d8 D. [Ar] 4s24p64d8 6.Which of the following is an excited-state electron configuration? A. 1s22s22p1 B. 1s22s22p63s1 C. 1s22s22p43s1 D. 1s22s22p63s23p64s1 7.Which one of the following corresponds to the electron configuration of the stable nitrogen ion? A. 1s2 B. 1s22s22p3 C. 1s22s22p6 D. 1s22s22p63s23p6 8.What mass of aluminum chloride can one obtain from the reaction of 6.00 mol of barium chloride? (The molar mass of AlCl3 is 133.3 g/mol) Al2(SO4)3 + 3BaCl2 3BaSO4 + 2AlCl3 9. For the reaction, 3A + 2B2 A3B4, in the drawing, what is the limiting reactant? 10. Consider the sine waves representing light or electromagnetic radiation. Which one corresponds to photons with the smallest energy? 11.What is the mole fraction of nitrogen N2 in a vessel that contains 0.39 atm of N2 and 0.11 atm of O2?











