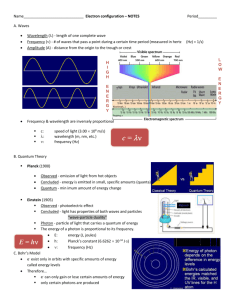
Quantum Numbers – Student Notes
advertisement
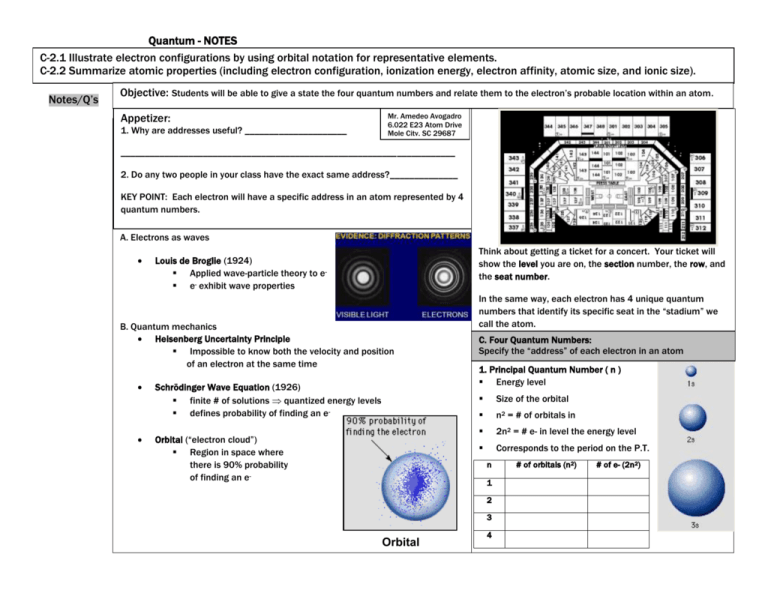
Quantum - NOTES C-2.1 Illustrate electron configurations by using orbital notation for representative elements. C-2.2 Summarize atomic properties (including electron configuration, ionization energy, electron affinity, atomic size, and ionic size). Notes/Q’s Objective: Students will be able to give a state the four quantum numbers and relate them to the electron’s probable location within an atom. Appetizer: 1. Why are addresses useful? _____________________ Mr. Amedeo Avogadro 6.022 E23 Atom Drive Mole City, SC 29687 UPPE _____________________________________________________________________ 2. Do any two people in your class have the exact same address?______________ KEY POINT: Each electron will have a specific address in an atom represented by 4 quantum numbers. A. Electrons as waves Think about getting a ticket for a concert. Your ticket will show the level you are on, the section number, the row, and the seat number. Louis de Broglie (1924) Applied wave-particle theory to e e- exhibit wave properties B. Quantum mechanics Heisenberg Uncertainty Principle Impossible to know both the velocity and position of an electron at the same time Schrödinger Wave Equation (1926) finite # of solutions quantized energy levels defines probability of finding an eOrbital (“electron cloud”) Region in space where there is 90% probability of finding an e- In the same way, each electron has 4 unique quantum numbers that identify its specific seat in the “stadium” we call the atom. C. Four Quantum Numbers: Specify the “address” of each electron in an atom 1. Principal Quantum Number ( n ) Energy level Size of the orbital n2 = # of orbitals in 2n2 = # e- in level the energy level Corresponds to the period on the P.T. n 1 2 3 Orbital 4 # of orbitals (n2) # of e- (2n2) Quantum - NOTES Notes/Q’s 2. Angular Momentum Quantum # ( l ) Energy sublevel Orbitals combine to form a spherical shape. Shape of the orbital 4. Spin Quantum Number ( ms ) Electron spin +½ or -½ An orbital can hold 2 electrons that spin in opposite directions. Pauli Exclusion Principle No two electrons in an atom can have the same 4 quantum numbers. Each e- has a unique “address”: 1. Principal # 3. Magnetic Quantum Number ( ml ) Orientation of orbital Specifies the exact orbital within each sublevel Each orientation can hold 2 electrons max 2. Ang. Mom. # energy level (1, 2, 3, 4, 5, 6, or 7) [Period] sublevel (s,p,d,f) 3. Magnetic # orbital 4. Spin # electron [block on P.T.] Sketch the shape of an s orbital and a p ortibtal. 1. How does a 2s orbital differ from a 1s orbital? 2. How do a 2px and a 2py orbital differ?