3.4 quantum numbers
advertisement

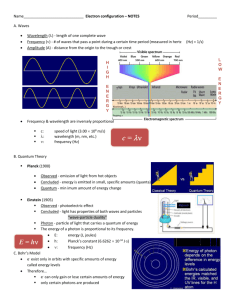
SCH 4U1 THE ORGANIZATION OF ELECTRONS: Quantum Numbers Electrons can be organized by four quantum numbers. This combination of numbers are unique for any given element. The first three numbers were created by Erwin Schrödinger and the last number was created by Wolfgang Pauli. The principal quantum number (n), introduced by Bohr is a whole number (1,2,3...) that specifies the energy level of an atomic orbital and its relative size. The greatest number of electrons allowed at any energy level is 2n2. The secondary quantum (l), describes an orbital’s shape. The numbers range from 0 to n-1. An s sublevel = 0, a p sublevel = 1, a d sublevel = 2, an f = 3, g = 4, h = 5...etc. The number n will give the number of sublevels. The number n2 will give the number of orbitals at that energy level. The magnetic quantum number, ml, indicates the number of orientations a sublevel may have. They are numbers from –l to +l. Sublevel Secondary quantum number, l Number of orientations Magnetic quantum number, ml s p d f g The fourth and last number is the spin quantum number, ms, and it describes the spin of an electron. In order to get two electrons into the same orbital the electrons must have opposite spin. There are only two values: -1/2 and +1/2. With all four quantum numbers any electron is unique and can be described. Identify the electron qualities of the following quantum numbers: n=4 l=1 ml = -1 ms = +1/2 n=2 l=0 ml = 0 ms = -1/2