Chemical - carbonation
advertisement

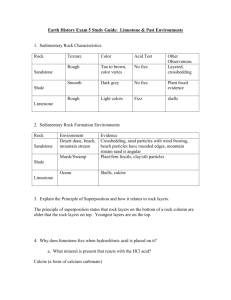
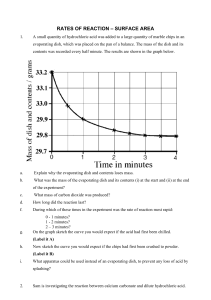
Carbonation in Chemical Weathering Chemical weathering is caused by rain water reacting with the mineral grains in rocks to form new minerals (clays) and soluble salts. These reactions occur particularly when the water is slightly acidic. These chemical processes need water, and occur more rapidly at higher temperature, so warm, damp climates are best. Chemical weathering is the first stage in the production of soils. Carbonation, sometimes known as solution, is the process of acid rain dissolving the rock face, causing the rock to decay and in some cases forming caves. The process starts when carbon dioxide in the air dissolves in rainwater and becomes weakly acidic. Carbonic acid once in contact with calcium carbonate produces calcium hydrogen carbonate (also known as calcium bicarbonate). The calcium bicarbonate which already is dissolved in water is removed rapidly leaving only impurities of the rock behind. As the acid rain seeps into cracks and cavities, over many years, carbonation of the rock can form spectacular cave systems. Here is the chemical equation that occurs when carbonation takes place... CO2 + H2O -> H2CO3 carbon dioxide + water -> carbonic acid H2CO3 + CaCO3 -> Ca(HCO3)2 carbonic acid + calcium carbonate -> calcium bicarbonate Also, stalagmites (on the ground) and stalactites (on the roof) form in caves as water drips from the roof, depositing some of its dissolved calcium carbonate, as carbon dioxide is released into the air. The picture below is an example of a cave formed by carbonation, with both stalagmites and stalactites present... By Zak Flesch 10N