Earth History Exam 5 Study Guide: Limestone & Past Environments
advertisement

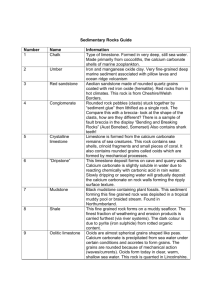
Earth History Exam 5 Study Guide: Limestone & Past Environments 1. Sedimentary Rock Characteristics: Rock Texture Color Acid Test Rough Tan to brown, color varies No fizz Smooth Dark grey No fizz Plant fossil evidence Rough Light colors Fizz shells Sandstone Shale Other Observations Layered, crossbedding Limestone 2. Sedimentary Rock Formation Environments Rock Sandstone Environment Desert dune, beach, mountain stream Marsh/Swamp Evidence Crossbedding, sand particles with wind frosting, beach particles have rounded edges, mountain stream sand is angular Plant/fern fossils, clay/silt particles Ocean Shells, calcite Shale Limestone 3. Explain the Principle of Superposition and how it relates to rock layers. The principle of superposition states that rock layers on the bottom of a rock column are older that the rock layers on top. Youngest layers are on the top. 4. Why does limestone fizz when hydrochloric acid is placed on it? a. What mineral is present that reacts with the HCl acid? Calcite (a form of calcium carbonate) b. Explain the chemical reaction that occurs to cause the fizzing. The HCl reacts with the calcite to create carbon dioxide bubbles (the fizzing) 5. What are two ways that calcium carbonate can form in an ocean environment? a. The calcium in the bones, shells, and scales of dead animals combines with carbon and oxygen. b. The carbon dioxide (CO2) exhaled by organisms reacts with calcium in the water. 6. Explain the process of limestone formation. • • • Calcium carbonate does not dissolve in water, so if it forms in the sea or falls into the sea, it will sink to the bottom. The calcium carbonate acts as a matrix and forms a layer at the bottom of the ocean. Over time through compaction the layer turns into limestone. 7. What is a fossil? • • A fossil is any remains, trace, or imprint of a plant or animal that has been preserved in the earth’s crust since some past geological or prehistoric time. Any evidence of past life