Lab 7: UV-Vis/Fluorescence
advertisement

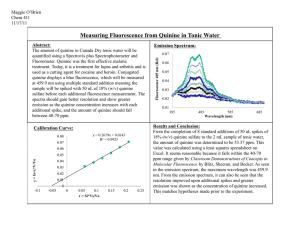
UV/VIS, Fluorescence Lab Report Introduction: Ultraviolet and Visible spectroscopy is the absorption of atoms and molecules in the UV/VIS spectral region. The absorption in the visible range directly affects the perceived color of the chemicals involved and by undergoing electronic transitions. The UV region is scanned from 200-400nm while the visible region is 400-800nm. When comparing spectra of different compounds, we use Beer’s Law to calculate the absorption values. Sometimes the equation can be used to measure the molar absorptivity of a specific molecule. Some applications of the UV/VIS are the quantitative determination of transition metals, organic compounds, and the biological macromolecules. Fluorescence spectroscopy is almost the opposite of what UV/VIS is. Fluorescence spectroscopy measures the fluorescence of a sample, through a fluorometer, where it is the transition of molecules from the excited state to the ground state, where they are excited at a certain wavelength and emit energy at a different wavelength. Some applications of fluorescence include the analysis of organic compounds for biochemical, medical, and chemical research purposes. Purpose: The purpose of this experiment was to determine the calibration and concentrations of salicylic acid using ultra violet and visible light radiation and quinine using the fluorescence as well as the unknown concentrations for pure salicylic acid, aspirin, and tunic water. Methods: Day 1: For the first day we worked with the UV/VIS. We measured various concentrations of the salicylic acid dissolved in hexane. Samples were made from a 1000ppm stock and we made standards of 40 with 4ml, 25 with 2.5ml, 10 with 1ml, and 2 with .2ml ppm solutions in 100ml volumetric flasks. We also made samples of the salicylic acid in hexane as well as pure powder salicylic acid as well as aspirin samples. We did three runs of each standard and samples and recorded the absorbance where each standard had the same wavelength in common. For us the wavelength was 240 nm and we recorded the absorbance at this wavelength for each run and created a calibration curve to determine the concentration of unknowns. Day 2: This day we worked on the Fluorescence spectroscopy and as with the UV/VIS we made standards of quinine from a 1000ppm stock in sulfuric acid (H2 SO4 ) and used tunic water as our sample to determine the level of quinine in the water. Our standards were 10 with 5ml, 5 with 2.5ml, 1 with .5ml, and .1ppm with .05ml of quinine in 50ml volumetric flasks with the H2 SO4 . We did three runs of each standard and sample and recorded the intensity and wavelength from the largest peak. Our optimal excitation wavelength was 345nm and optimal emission wavelength was 449nm. We calculated a calibration curve for this also to determine our unknown concentrations. Results: We recorded the wavelength and absorbance for the UV/VIS standards and unknowns and used an equation to determine the unknown concentrations. The average absorbance was calculated for the three runs to give us more accurate results and the optimal wavelength was 240nm. Concentration (ppm) 2 10 25 40 Aspirin Unknown 1 Absorbance .102 .126 .469 .724 1.436 .127 The calibration equation was y = 55.7017x - .538; the unknown concentration was 6.54ppm and the aspirin was 79.45ppm. We also calculated the % error for each and the unknown % error was 6.62% and the aspirin was between 74.19 and 84.71% based on our calculated error from the other sample. The Fluorescence data of quinine is provided below: Concentration (ppm) .1 1 5 10 Tunic Water Intensity 90.6 150.3 40 60.3 14.6 The calibration equation for fluorescence was y = -6.717x +112.37; the tunic water quinine concentration is 13.8ppm, which falls in our standard concentration range. Conclusion: Overall both experiments went without complications. However for the UV/VIS there were some problems with dissolving the salicylic acid in the hexane as well as the aspirin. So the results probably came out different because not all the light was absorbed since not everything was dissolved. Some other sources of error could be from not all the standards having the 240nm peak so the absorbances were not as accurate. Fluorescence worked also but it had its own problems as well. Some sources of error for this included the inability to accurately make the .5ml and .05ml solutions of quinine in H2 SO4 because the amount was so small that we had to “eyeball” the measurements. The intensities for these standards were off from the rest because of the measurement problems. The concentration of the tunic water was calculated and it turns out that the concentration of 13.8ppm was within the FDA standards of quinine. On the other hand it is a lower concentration because the sample tunic water that was used expired in 2008, so the results weren’t as reliable for any current samples. Also if there was a newer sample used, there would most likely be a higher concentration of quinine in the tunic water because of the original manufacturing date for sale.