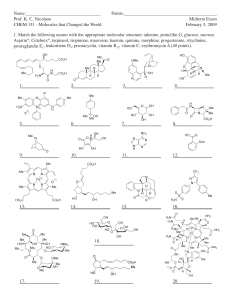
UV/VIS Review Questions a. Explain in context to what n, Π, Π
advertisement

UV/VIS Review Questions a. Explain in context to what n, Π, Π * stand for and using energy level diagrams why n to Π* transitions occur at higher wavelengths then Π to Π * transitions. b. Give an example of a molecule whose wavelength maximum will blue shift when it is analyzed as an aqueous solution after being analyzed as a hexane solution. Why does this occur? Explain IN DETAIL and make sure to use energy level diagrams. c. When you report UV-Vis wavelengths for your solution, you report wavelength maxima even though the peak spans many wavelengths. These peaks are broad and Gaussian. What causes these broad peaks and what do they prevent you from detecting (seeing)? d. Penicillin is a common antibiotic given to treat bacterial infections. Sometimes you are given penicillin, sometimes you are given amoxicillin, and sometimes you are given ampicillin. You have an infection and you find an old bottle of medication in your medicine cabinet and all that you can read is “cillin”. Devise a UV/Vis experiment to determine without reservation which of the “cillins” are in that bottle. Make sure to comment on solvents you will use, type of detector you will use, and the predicted wavelength maxima. Penicillin amoxicillin ampicillin Some preliminary experiments that you did determined that, at your wavelength maximum for penicillin, an absorbance of 0.6 gives you a concentration of 10ppm penicillin and an absorbance of 0.3 gives you a concentration of 5ppm penicillin. Explain how this information can be used to help you with your experiment. e. Determine the UV wavelength maximum for each of the following molecules O O OH Cl f. Use the following information to determine how much Chromium (expressed in pph) is in your original solution (10.00 mL) if a diluted solution of volume 1.000 L measures an absorbance of 0.09. Standards 1 2 3 4 Concentration (µg/mL) 1 6 15 34 % Absorption 1 13 30 55