Ac Bio Chp 2.2 Review WS
advertisement

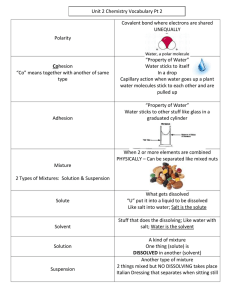
Mr. Sheehan’s Pd Name Ac Bio Chp 2.2 Review WS 1. Water is polar. What does that mean? Draw a water molecule showing where the positive and negative charges are. Polar = unequal charge 2. Water forms ____hydrogen__________________ bonds between a hydrogen atom on one molecule and the ____oxygen___________________ atom of ___another___________________ molecule. 3. Define cohesion and adhesion. Give an example of each. Cohesion – attraction between molecules of the same substance. together to fill a cup over the brim Ex: Water molecules sticking Adhesion –attraction between molecules of different substances. Ex: Water sticking to a cup/climbing up the sides 4. The amount of energy required to raise the temperature of a substance is its __heat capacity__. 5. A. Define mixture. physical combination of two/more things – NO chemical change B. What is the difference between a compound and a mixture? Give an example of each. Compound = chemical combination of two/more elements Ex – H2O Mixture = physical combination of two/more things Ex – Salt water, Cran-apple juice 6. What is the difference between a solution and a suspension? Give an example of each. Solution = evenly mixed mixture Ex – Salt water Suspension = mixture where substances are not dissolved Ex – Oreos in milk, blood 7. I pour chocolate syrup into my milk. What is the solvent and what is the solute? Solvent = milk Solute = chocolate syrup 8. ____Water______________ is the universal solvent. FLIP TO THE BACK FLIP TO THE BACK Mr. Sheehan’s Pd Name 9. The pH scale measures the concentration of __H+_________ ions. 10. Draw a pH scale. Indicate the following: A. Number range 2. Neutral III. Labeled arrow showing increasing acidity and another arrow for increasing basicity. Base 11. Acids form __H+_______ ions. Bases form __OH-_______ ions. 12. ___Buffer___________ - a weak acid or base that can react with strong acids or bases to prevent changes in pH. 13. If I have a strong acid (pH = 2), I would use a base buffer with a pH of __12-14______. 14. Why are buffers important to organisms? Help maintain homeostasis