Ch 8 Key Terms Cloze
advertisement
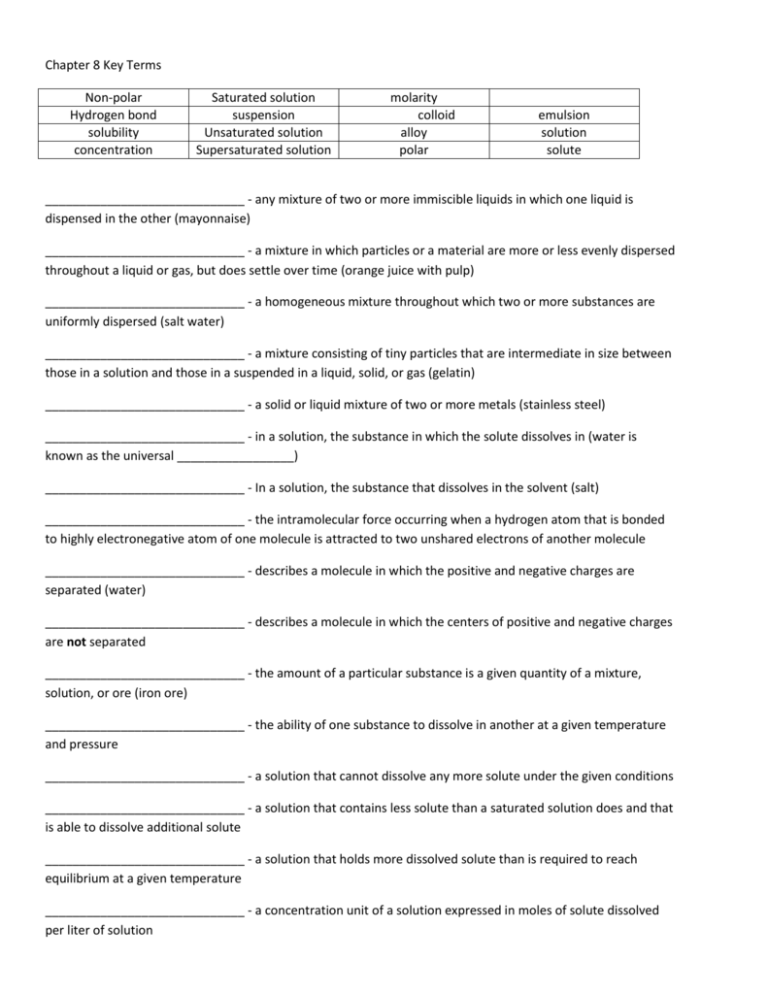
Chapter 8 Key Terms Non-polar Hydrogen bond solubility concentration Saturated solution suspension Unsaturated solution Supersaturated solution molarity colloid alloy polar emulsion solution solute _____________________________ - any mixture of two or more immiscible liquids in which one liquid is dispensed in the other (mayonnaise) _____________________________ - a mixture in which particles or a material are more or less evenly dispersed throughout a liquid or gas, but does settle over time (orange juice with pulp) _____________________________ - a homogeneous mixture throughout which two or more substances are uniformly dispersed (salt water) _____________________________ - a mixture consisting of tiny particles that are intermediate in size between those in a solution and those in a suspended in a liquid, solid, or gas (gelatin) _____________________________ - a solid or liquid mixture of two or more metals (stainless steel) _____________________________ - in a solution, the substance in which the solute dissolves in (water is known as the universal _________________) _____________________________ - In a solution, the substance that dissolves in the solvent (salt) _____________________________ - the intramolecular force occurring when a hydrogen atom that is bonded to highly electronegative atom of one molecule is attracted to two unshared electrons of another molecule _____________________________ - describes a molecule in which the positive and negative charges are separated (water) _____________________________ - describes a molecule in which the centers of positive and negative charges are not separated _____________________________ - the amount of a particular substance is a given quantity of a mixture, solution, or ore (iron ore) _____________________________ - the ability of one substance to dissolve in another at a given temperature and pressure _____________________________ - a solution that cannot dissolve any more solute under the given conditions _____________________________ - a solution that contains less solute than a saturated solution does and that is able to dissolve additional solute _____________________________ - a solution that holds more dissolved solute than is required to reach equilibrium at a given temperature _____________________________ - a concentration unit of a solution expressed in moles of solute dissolved per liter of solution Answer Key 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. Emulsion Suspension Solution Colloid Alloy solvent Hydrogen bond Polar molecule Non-polar molecule Concentration Solubility Saturated Unsaturated Supersaturated molarity









