Unit 2 Chemistry Vocabulary Pt 2 UNEQUALLY Polarity
advertisement
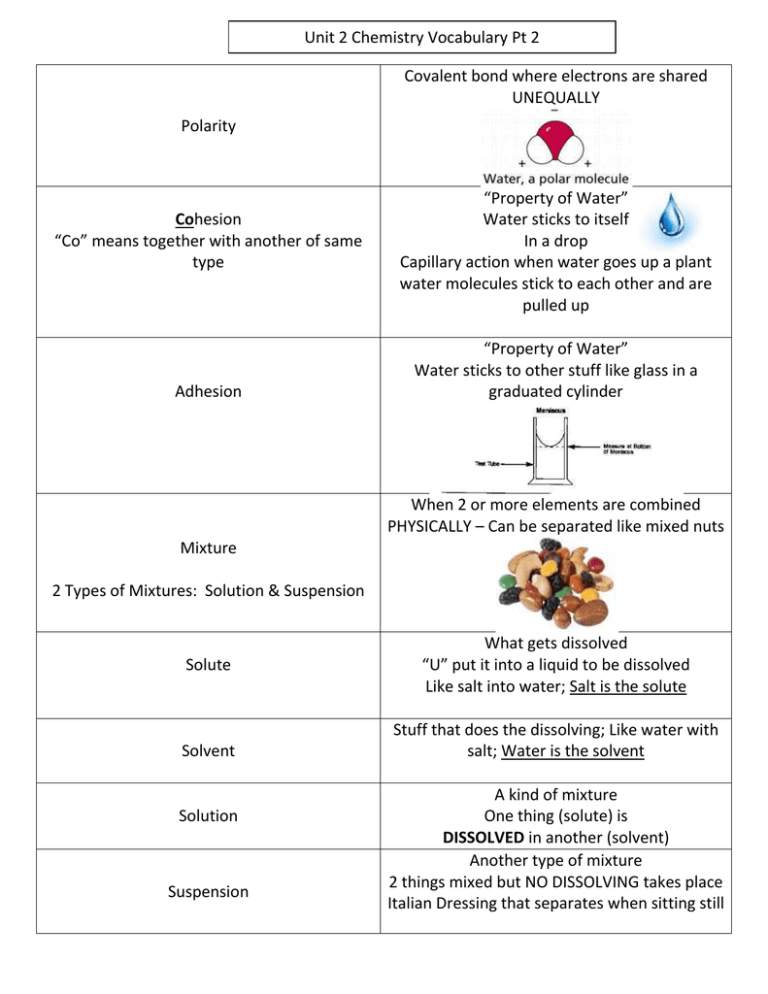
Unit 2 Chemistry Vocabulary Pt 2 Covalent bond where electrons are shared UNEQUALLY Polarity Cohesion “Co” means together with another of same type Adhesion “Property of Water” Water sticks to itself In a drop Capillary action when water goes up a plant water molecules stick to each other and are pulled up “Property of Water” Water sticks to other stuff like glass in a graduated cylinder When 2 or more elements are combined PHYSICALLY – Can be separated like mixed nuts Mixture 2 Types of Mixtures: Solution & Suspension Solute Solvent Solution Suspension What gets dissolved “U” put it into a liquid to be dissolved Like salt into water; Salt is the solute Stuff that does the dissolving; Like water with salt; Water is the solvent A kind of mixture One thing (solute) is DISSOLVED in another (solvent) Another type of mixture 2 things mixed but NO DISSOLVING takes place Italian Dressing that separates when sitting still Measure of how much H+ ions are in solution [H+] = concentration of H+ ions H2O H+ and OH Acid Base pH Acids High [H+] Increase in H+ ions = pH decreases Bases Low [H+] Decrease in H+ ions = pH increases What is it? Weak acid or base What does it do? Reacts with strong acid or base to neutralize the conditions and maintain pH balance Why? Homeostasis (pH Balance) Buffers Strong Acid (0-3) Weak Acid (Neutral) Weak Base (4-6) (7) (8-10) Strong Base (11-14)











